Salts
A salt is a chemical substance formed when the hydrogen ions in an acid are replaced by a metal or ammonium ions,
Types of salts
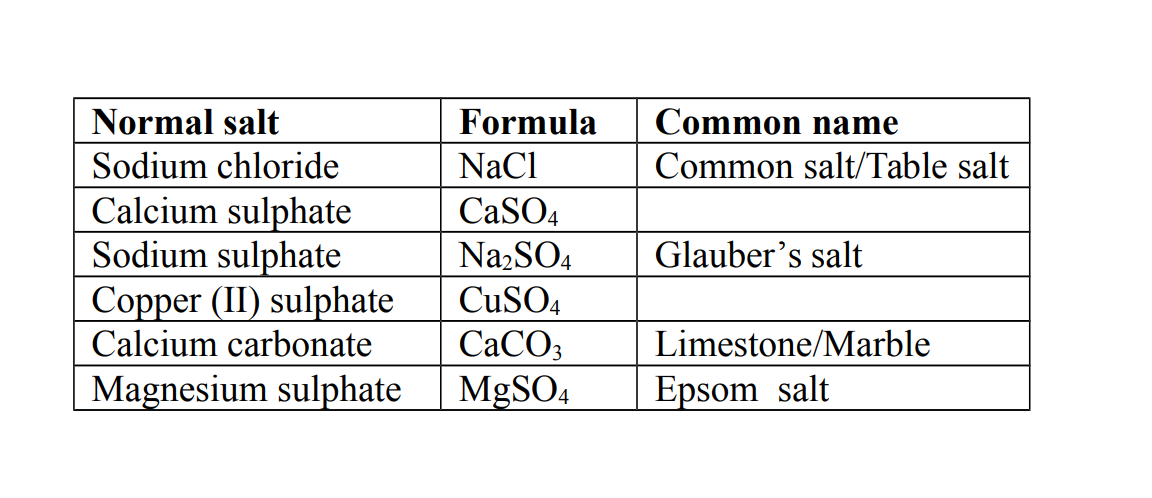
1. Normal salt
This is a salt formed when all the hydrogen ions in an acid are replaced by a metal or ammonium ions.
Example of Normal Salts
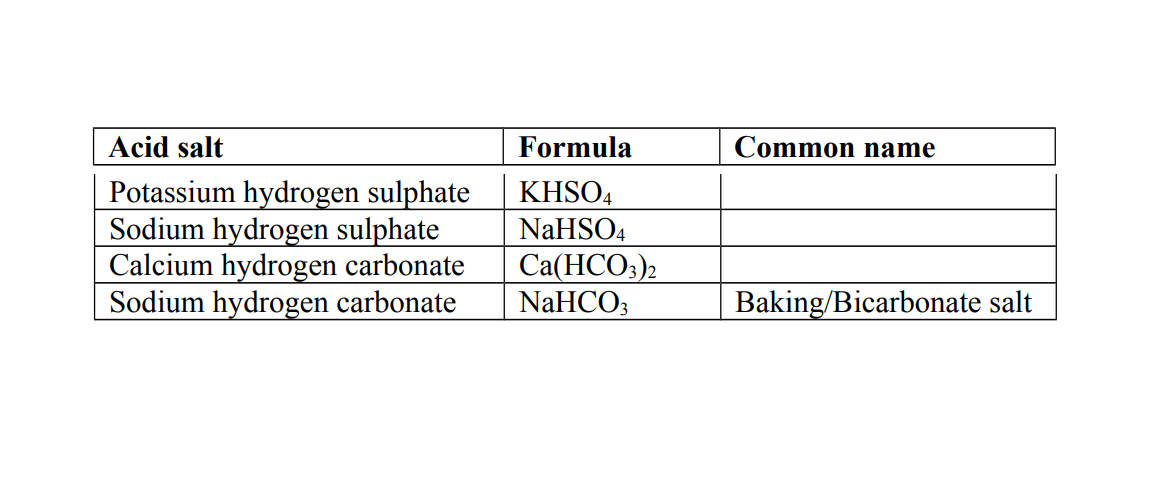
2. Acid salt
This is a salt formed when part of the hydrogen ions in an acid are replaced by a metal or ammonium ions.
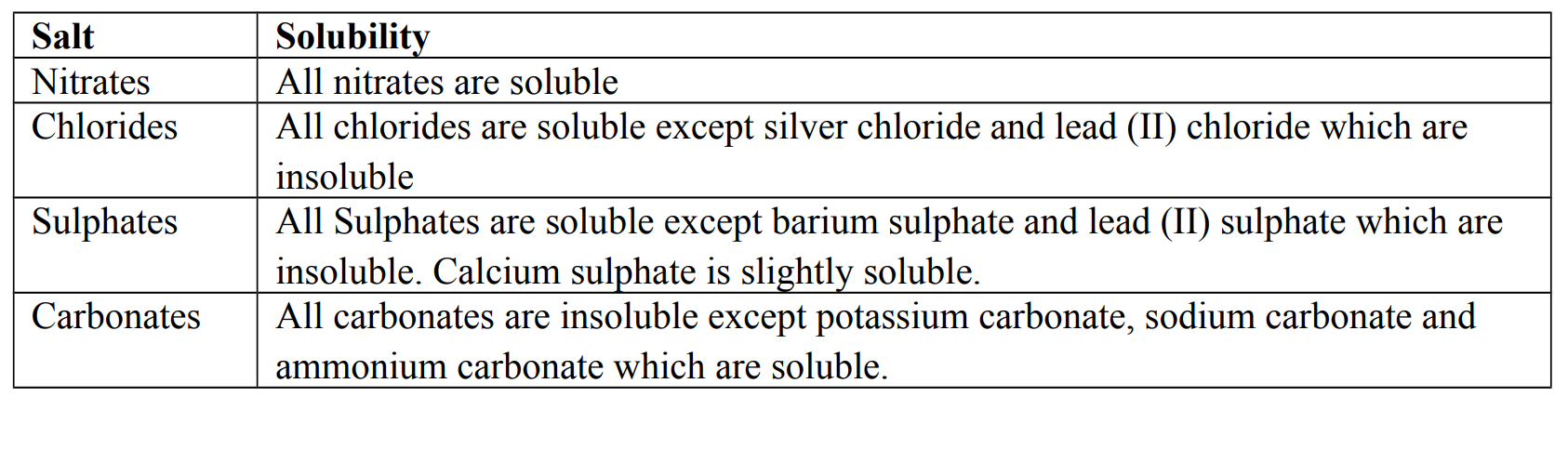
Solubility of salts
Solubility is the ability of a salt to dissolve in water.
Facts about solubility of salts in water
Preparation of salts
The method chosen to prepare a given salt depends on its solubility and how it can be separated from the mixture of other products.
Methods of preparing salts
- Neutralization
- Replacement (Displacement)
- Synthesis
- Precipitation (Double Decomposition)
Neutralization
It is a reaction between an acid and a base to form a salt and water only.
Acid + Base → Salt + WaterNeutralization includes the preparation of a salt by reacting:
(a) a metal hydroxide with a dilute acid.
(b) an insoluble metal oxide with a dilute acid.
(c) a metal carbonate with a dilute acid.
Preparation of soluble salts by neutralization
Metal hydroxide + Acid → Salt + WaterPreparation of sodium chloride
Reagents- Sodium hydroxide, NaOH
- Hydrochloric acid, HCl
- Sodium chloride, NaCl
- Water, H2O
Method of preparation salt step by step
A preliminary titration is carried out to find the end point with the help of an indicator.
- Using a pipette, measure 25.0cm3 of Sodium hydroxide and put it into a conical flask.
- Add two or three drops of indicator to sodium hydroxide using a teat pipette.
- Fill the burette to the zero reading with dilute hydrochloric acid
- Place the conical flask on a white tile below the burette.
- Add dilute hydrochloric acid from the burette to sodium hydroxide in the conical flask, while swirling, until the solution just changes colour.
- From the titration result, we can know the exact volume of hydrochloric acid needed to react with 25.0cm3 of sodium hydroxide.
Hydrated salt
This is a salt that contains water of crystallization. They contain a fixed amount of water in their crystal lattice. This is called water of crystallization. The water of crystallization is part of the structure. If this water is removed, by heating for example, the colour and shapes of the crystals may change.
Examples of hydrated salts
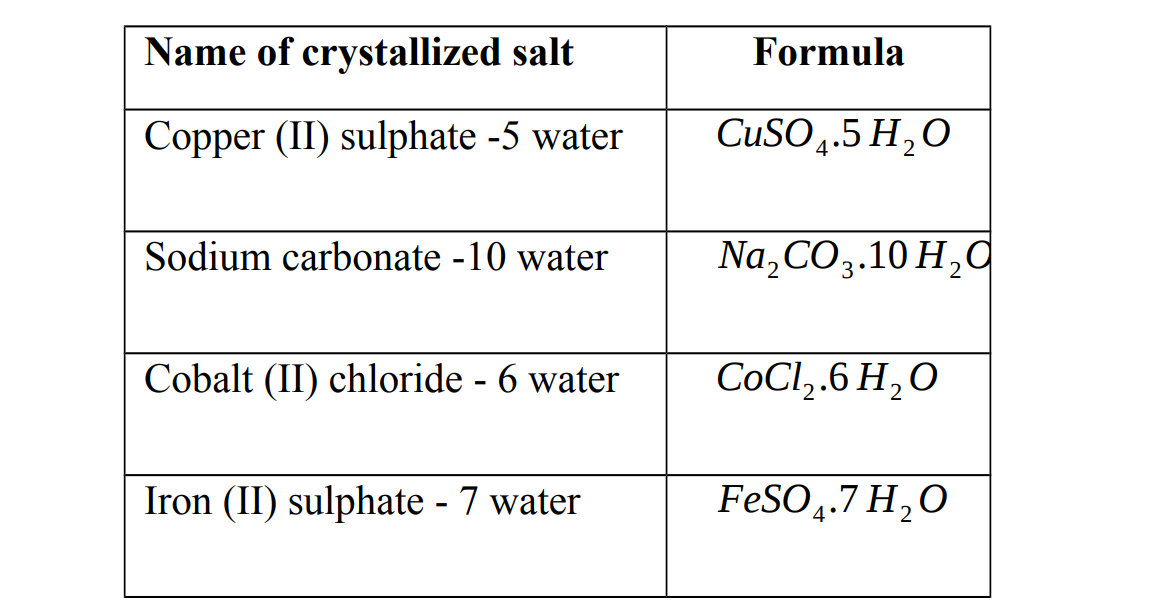
Anhydrous salts
A salt which has lost its water of crystallization is called an anhydrous salt .
When water is added to an anhydrous salt, the salt becomes hydrated.
For example, when blue copper (II) sulphate crystals are heated, stem is produced and a pale- blue or white powder.
When water is added to anhydrous copper (II) sulphate heat is produced and a blue solution is formed: This process is called hydration .
Efflorescence
This is the loss of water of crystallization to the atmosphere. an example is Crystals of sodium carbonate - 10- water become Powderly when exposed to air
Deliquescence
This is the absorption of water from the atmosphere to form a solution. Calcium chloride is a deliquescent salt. It is used as a drying agent in desiccators. A desiccator is a piece of equipment used to dry substances.
Hygroscopic
A hygroscopic substance absorbs water from the air but does not the change its state. Anhydrous cobalt chloride is a hygroscopic salt. Water changes anhydrous cobalt chloride from blue to pink.
This reaction is often used as a test for the presence of water. The process can be reversed by heating the pink hydrated salt:
Concentrated sulphuric acid is also hygroscopic. It can be used to dehydrate blue crystals of hydrated copper (II) sulphate forming the pale blue anhydrous salt.
Replacement (Displacement)
This is a method where the hydrogen ions in an acid are replaced by a metal. It can also be defined as a reaction in which one element displaces another from a compound.
Reactive metal + Acid → Salt + HydrogenPreparation of iron (II) sulphate
- Add iron fillings to warm dilute sulphuric acid in a beaker until no more hydrogen gas is evolved.
- Filter off the solution when the reaction is complete to remove excess iron.
Note Iron should be in excess so that all the acid is used up. Air must be excluded to prevent oxidation of iron (II) sulphate.
Synthesis
This method involves the direct combination of elements for binary salts.
In this method, a salt is prepared directly from its elements i.e. a metal and a halogen.
Metal + Halogen → SaltExample of Synthesis is the Preparation of iron (II) chloride
Iron (II) chloride can be prepared by passing chlorine gas over heated iron.
Precipitation
Precipitation is the formation of an insoluble product and may occur on mixing two solutions.
Precipitation is an example of double decomposition. In double decomposition, two solutions are mixed to form an insoluble salt and a soluble solution.
Soluble salt + soluble salt → insoluble salt + soluble salt Soluble solution + soluble solution → insoluble solid + soluble solutionIn double decomposition reactions, cations and anions are exchanged.
Precipitation is also an example of ionic association which is the attraction of oppositely charged ions to one another to form a solid called precipitate abbreviated as ppt.
Preparation of insoluble salts by precipitation (Double decomposition)
Preparation of silver chloride
Reagents- Silver nitrate, AgNO3
- Sodium chloride, NaCl (Alternatively hydrochloric acid, HCl)
- Silver chloride, AgCl
- Sodium nitrate, NaNO3
Method of preparing silver chloride step by step
- Mix silver nitrate solution with sodium chloride solution in a beaker.
- A white precipitate of silver chloride forms.
- Allow the precipitate to settle.
- Filter off the precipitate and wash it with distilled water to remove any amount of sodium nitrate left.
- Dry the precipitate on the filter paper.
- A pure dry sample of silver chloride forms.
Reduction and oxidation
Reduction and oxidation can be defined in terms of oxygen, hydrogen, electrons and oxidation number.
Oxidation in terms of oxygen
Oxidation is the addition of oxygen to a chemical substance.
Example Magnesium has been oxidized to magnesium oxide since oxygen has been added.
Iron (II) oxide (FeO) has been oxidized to iron (III) oxide (Fe2O3) since oxygen has been added.
Oxidation in terms of hydrogen
Oxidation is the removal of hydrogen from a chemical substance.
Example Ammonia (NH3) has been oxidized to nitrogen (N2) since hydrogen has been removed.
Hydrogen sulphide (H2S) has been oxidized to sulphur (S) since hydrogen has been removed.
Oxidation in terms of electrons.
Oxidation is the loss of electrons from a chemical substance.
Example Copper atoms (Cu) have been oxidized to copper (II) ions (Cu2+) since two electrons have been lost.
Aluminum atoms (Al) have been oxidized to aluminum ions (Al3+) since three electrons have been lost.
Chloride ions (Cl-) have been oxidized to chlorine molecules (Cl2) since electrons have been lost.
Oxidation number
Rules to consider when assigning the oxidation number
- The oxidation number of neutral particles like atoms and molecules is equal to Zero.
- The oxidation number of hydrogen in all compounds except metallic halides is +1
- The oxidation number of oxygen in all compounds except in peroxides and in OF2 is -2.
- For some metals the oxidation number is equal to the group number on the periodic table or the valence.
- The oxidation number of a simple ion is equal to the charge it carries.
- In neutral compounds, the sum of individual elements present is equal to zero.
- The sum of all oxidation numbers of all elements in a complex ion is equal to the charge on the ion.
Oxidation in terms of oxidation number
Oxidation is the increase in the oxidation number of a substance.
Example Copper atoms (Cu) have been oxidized to copper (II) ions (Cu2+) because the oxidation number has increased from 0 in Cu to +2 in Cu2+.
A Chloride ion (Cl- ) has been oxidized to chlorine atom (Cl) since the oxidation number has increased from -1 in Cl- to 0 in Cl.
Sulphur (S) has been oxidized to sulphur dioxide (SO2) since the oxidation number has increased from 0 in S to +4 in SO4.
Oxidizing agents (oxidants)
An oxidizing agent is a chemical substance which brings about oxidation of another substance but end up being reduced.
Examples of oxidizing agents.
- Oxygen, O2
- Chlorine, Cl2
- Hydrogen peroxide, H2O2
- Potassium permanganate, KMnO
- Manganese dioxide (manganese (IV) oxide), MnO
- Concentrated Sulphuric acid, H2SO4
Characteristics of an oxidizing agent.
(a) Supplies or donates oxygen to another substance. Lead (II) oxide (PbO) is an oxidizing agent because it has donated oxygen to hydrogen
(b) Removes hydrogen from another substance. Chlorine (Cl2) is an oxidizing agent because it has removed hydrogen from hydrogen sulphide (H2S)
(c) Accept electrons from another substance.
(d) Increases the oxidation number of another substance.
Reduction
Reduction in terms of oxygen
Reduction is the removal of oxygen from a chemical substance.
Example Carbon dioxide (CO2) has been reduced to carbon monoxide (CO) since oxygen has been removed.
Reduction in terms of hydrogen.
Reduction is the addition of hydrogen to a chemical substance.
Example Nitrogen (N2) has been reduced to ammonia (NH3) since hydrogen has been added.
Reduction in terms of electrons
Reduction is the gain of electrons.
Example Copper (II) ions (Cu2+) have been reduced to copper atoms (Cu) by gaining two electrons.
Reduction in terms of oxidation number
Reduction is the decrease in the oxidation number of a chemical substance.
Example Zinc ions (Zn2+) have been reduced to Zinc atoms (Zn) since the oxidation number has decreased from +2 in Zn2+ to 0 in Zn.
Reducing agents (Reductants)
A reducing agent is a chemical substance which brings about reduction but end up being oxidized.
Examples of reducing agents.
- Hydrogen, H2
- Carbon monoxide, CO
- Carbon, C
- Ammonia, NH3
- Hydrogen sulphide, H2S
- Sulphur dioxide, SO2
Characteristics of a reducing agent.
(a) Removes oxygen from another substance. Carbon monoxide (CO) is a reducing agent because it has removed oxygen from iron (III) oxide (Fe2O3)
(b) Donates or supplies hydrogen to another sustenance. Hydrogen sulphide (H2S) is a reducing agent because it has donated hydrogen to chlorine.
(c) Donates electrons to another substance
(d) Decreases the oxidation number of another substance. e.g Zinc atom (Zn) is a reducing agent because it has caused the decrease in the oxidation number of copper (II) ions from +2 in Cu2+ to 0 in Cu.
Qualitative analysis tests
Identification of ions.
Test for anions in solution
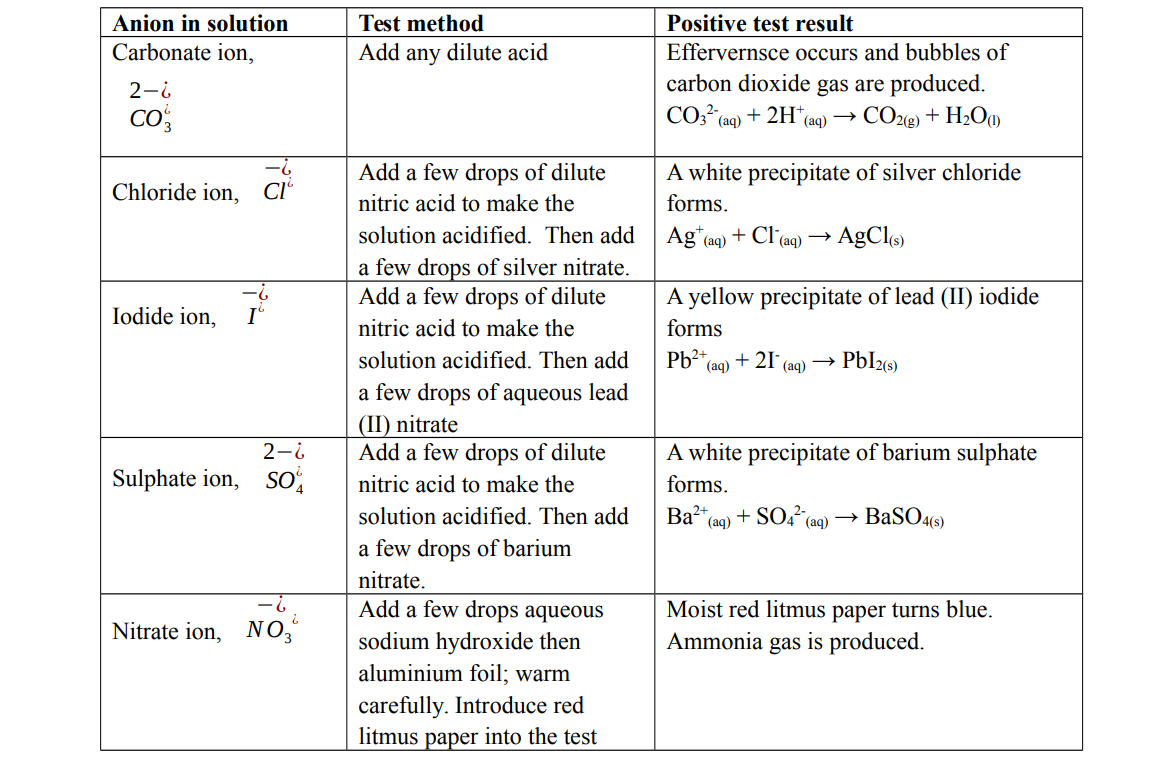
Test for cations in solution
When testing for a cation using either aqueous sodium hydroxide or aqueous ammonia, two observations will help identify the cation present:
- The colour of the precipitate formed on adding a few drops of chemical reagent.
- The solubility of the precipitate in excess chemical reagent
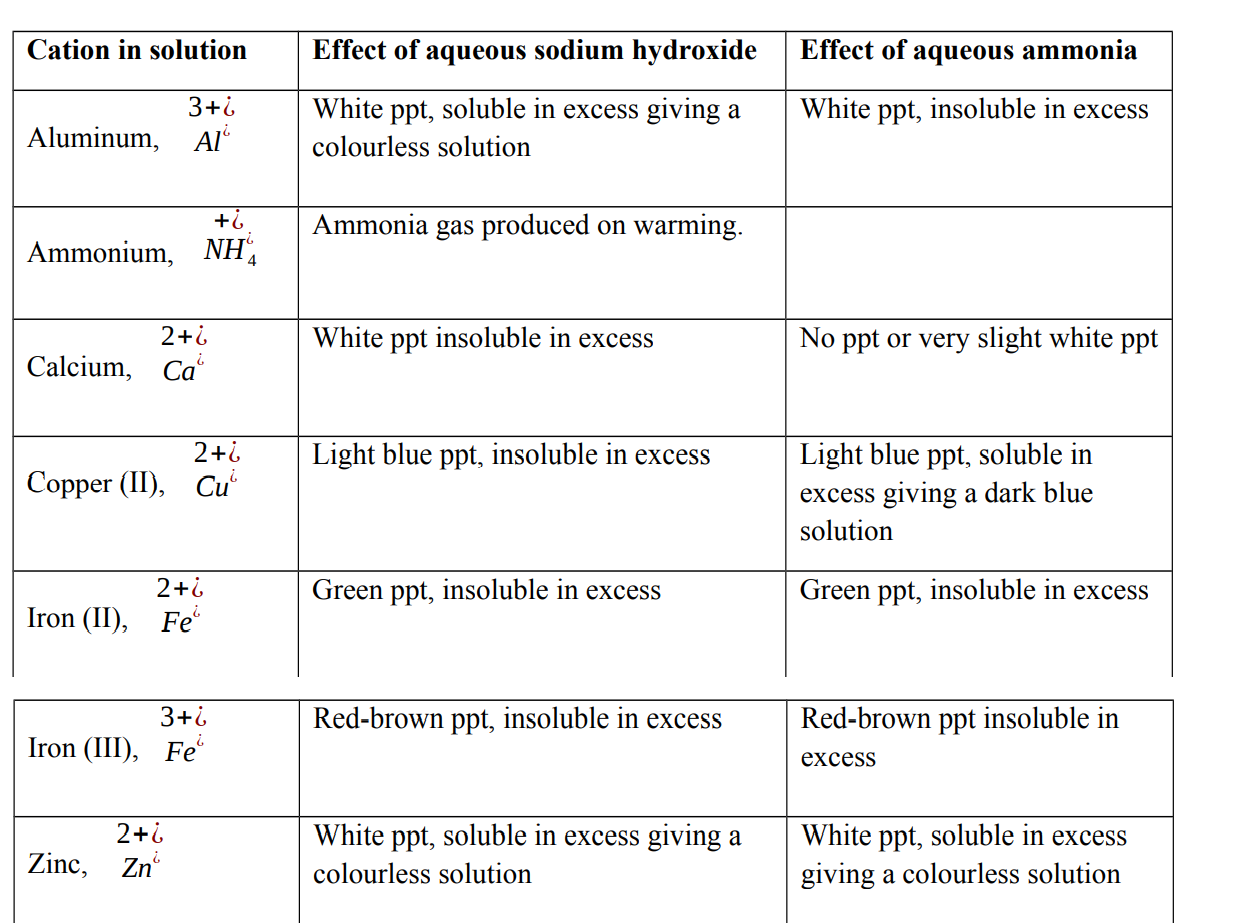
The cations react with hydroxide ions present in aqueous sodium hydroxide or ammonia to form insoluble hydroxides. These hydroxides appear as precipitates.
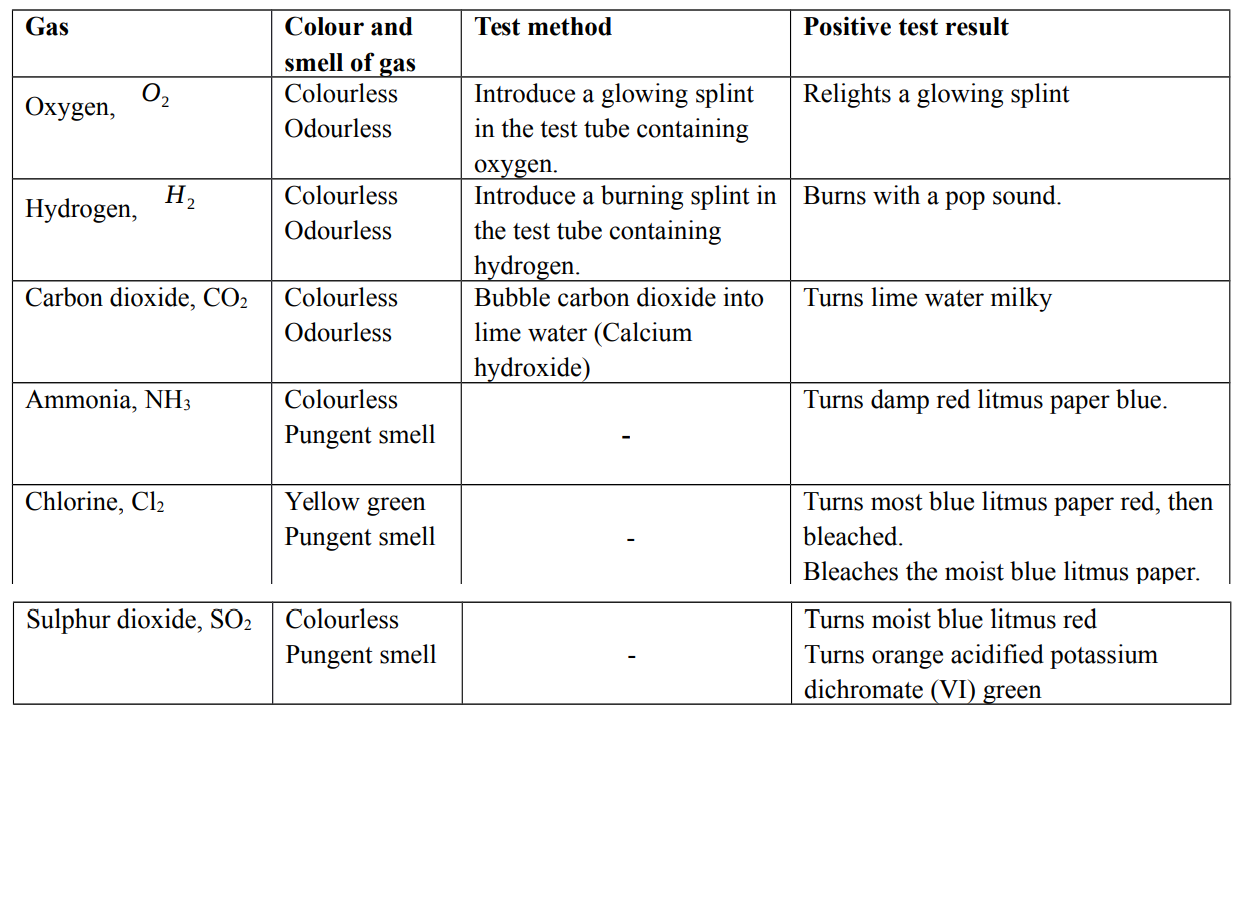
Test for gases
When recording observations for gases, it is important to record
- Presence of effervescences, if any
- Colour and smell of the gas
- Chemical test for the gas and test result
- Name of the gas