Matter and the kinetic theory
Matter is defined as is anything that occupies space and has mass.
Classify the basic units of matter
The particles found in matter are atoms ,molecules and ions
Classify the states of matter
- Solid; particles of a solid are closely packed and in constant vibration in their fixed position.
- Liquid; distance between particle of a liquid are far apart from each other as compared to the distance between solid particle and move about randomly.
- Gas ; the particles that make up a gas are very far from each other when compared to the distance of particle in liquids. This is the why gas is easily compressed.
Below are the three state of matter
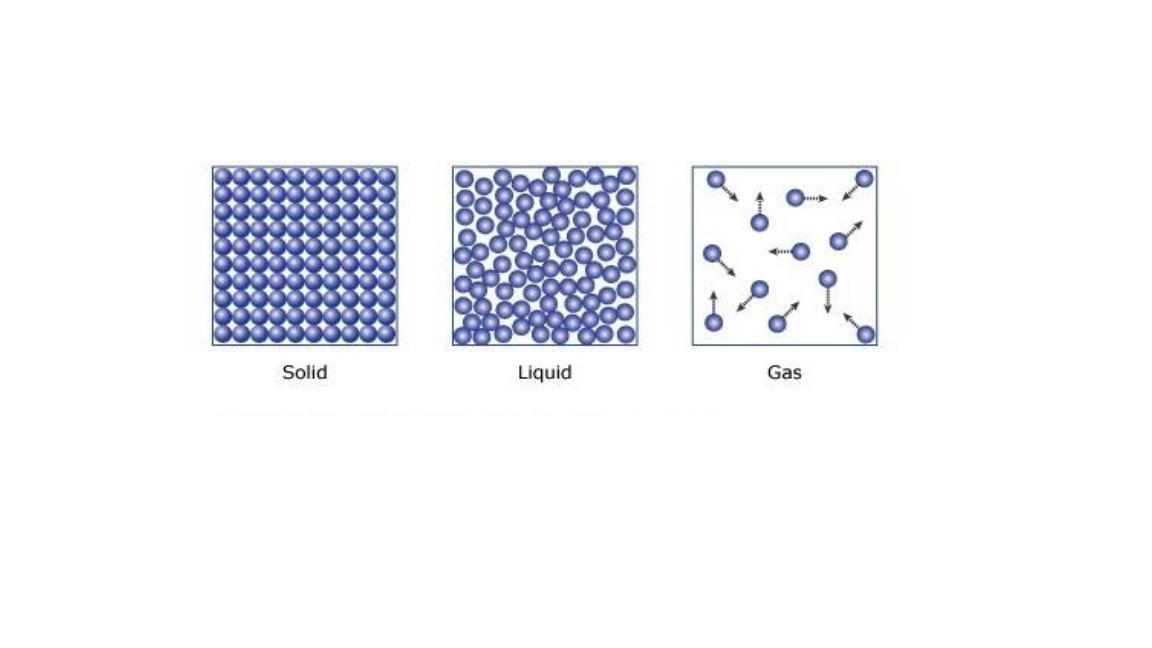
Characteristics from the three state of matter.
- SOLID : Solids have fixed volume and shape. To change the shape of a solid requires force, such as when it is broken or cut with an instrument. Example of Solids are Stone ,ice block, table salt, wood
- LIQUID : Liquids have fixed volume but not fixed shape. A liquid can change shape its shape and take on the shape of the container. Example of liquids are Water, paraffin, cooking oil
- GAS : Gas have no fixed shape and volume. A gas will expend to take the full volume of the container it is in. Example of gases are Oxygen ,carbon dioxide, chlorine gas
Changes of states of matter.
- Melting is the change of state from solid to liquid as a result of increase in temperature. The distance between particles increase
- Freezing point, is the change of state from liquid to solid. The distance between particles of the liquid reduce until solid state is achieved.
- Boiling point, is the temperature at which liquid changes to gas. The temperature remains constant at this point until all the liquid has changed to gas. The energy supplied to the liquid is used to increase the distance between the particles of the liquid.
- Condensation , is the change of state from gas to liquid.
- Sublimation , is the change of state from solid to gas or gas to solid.
The diagram below illustrate the change of matter
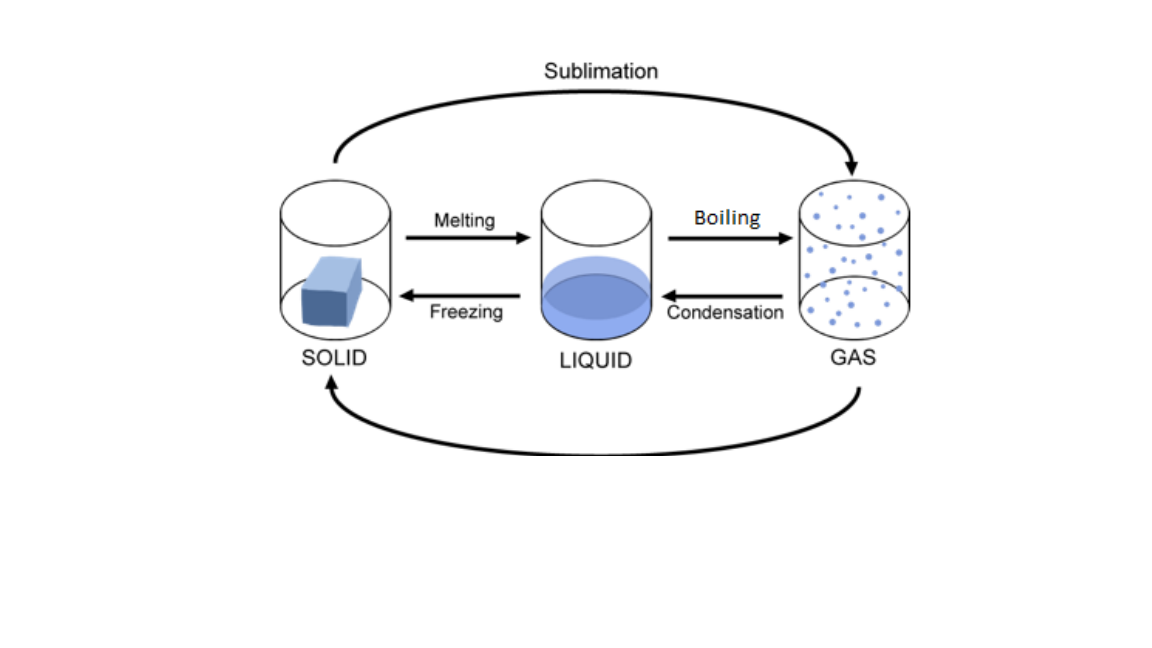
note that the absorption of heat and release of heat during changes of states of matter Changes the states of matter by:
Exothermic reaction , which is the release of heat during a reaction and endothermic reaction, absorption of heat during a reaction.
Diffusion
Is the movement of particles from region of higher concentration to region of lower concentration.
The factors that affect the rate of diffusion.
Molecular mass; the bigger the molecular mass the slower the rate of diffusion. The smaller the mass the faster the rate of diffusion.
Temperature; at higher temperature particles move faster hence faster rate of diffusion. At low temperature particles move slower hence the slower rate of diffusion. Therefore we can state that the rate of diffusion is directly proportional to temperature.
Concentration ; The higher the difference in concentration the faster the rate of diffusions. The lower the difference in concentration of the two substances the lower the rate of diffusion.