Oxygen
Oxygen gas exits as a diatomic molecule, O2. It makes up about 21% of air by volume
Preparation of oxygen
1. Laboratory preparation of oxygen
In the laboratory, oxygen gas can be prepared using the following chemicals:
(a) Potassium chlorate, KClO3
(b) Hydrogen peroxide, H2O2
(c) Sodium nitrate, NaNO3 and Potassium nitrate, KNO3
Potassium chlorate
Potassium chlorate is mixed with a catalyst manganese (IV) oxide upon heating and decomposes into potassium chloride and oxygen gas.
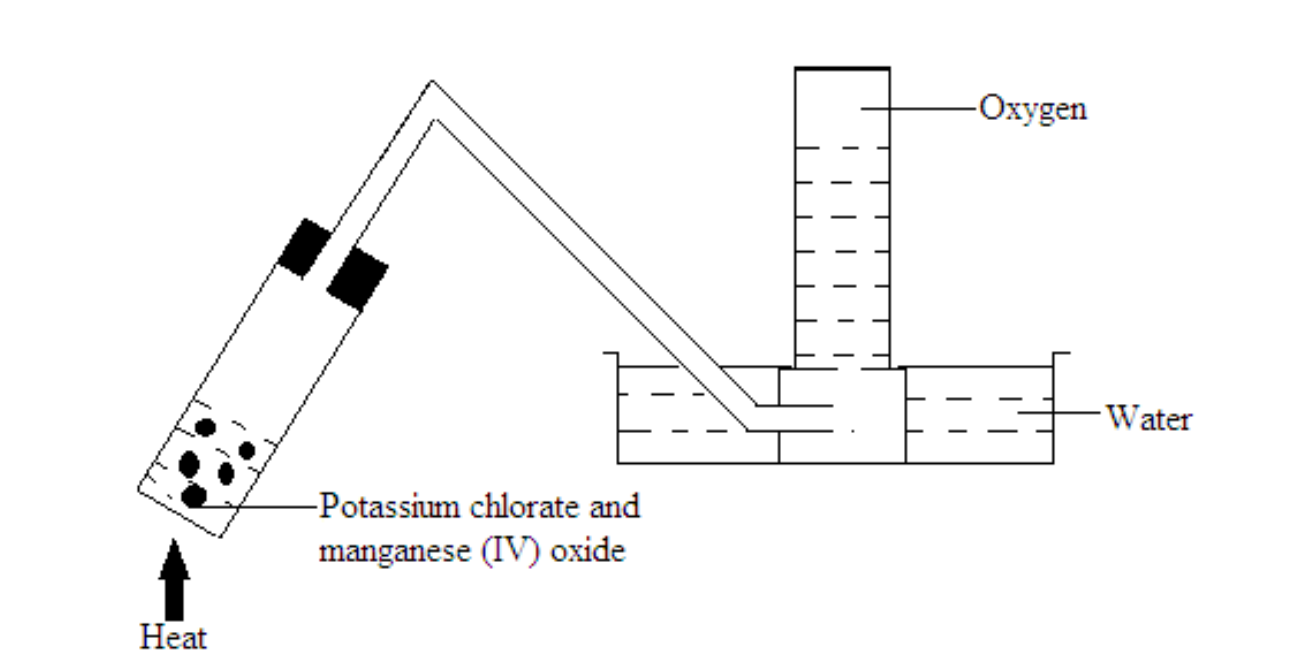
Note: This experiment is explosive
Hydrogen peroxide
Oxygen gas is also prepared by the decomposition of hydrogen peroxide solution using manganese (IV) oxide as a catalyst. No heating is required
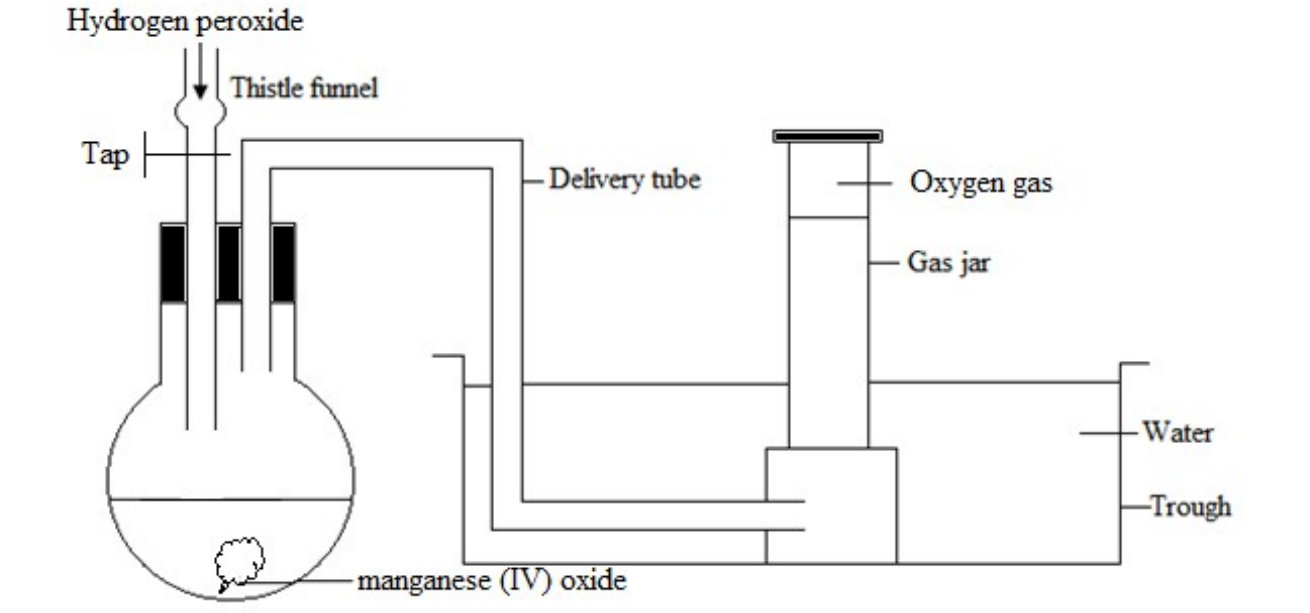
Oxygen gas is collected over water
Method of collection: Down ward displacement of water
Drying agent: Concentrated sulphuric acid
Sodium nitrate and potassium nitrate
Oxygen gas can be prepared by heating sodium nitrate or potassium nitrate
Industrial preparation of oxygen gas
On the large scale, oxygen gas is manufactured by fractional distillation of liquid air. Liquid oxygen boils at -183oC while liquid nitrogen boils at -196oC Nitrogen gas which has a the lower boiling point distils off first
Test for oxygen gas
Oxygen gas relights a glowing splint introduced in its container
Uses of oxygen
- It is used in the manufacture of steel in the blast furnace
- It is used in oxygen tents in hospitals for patients in the intensive care unit
- It used for welding in the oxy-acetylene flame
- It used by deep sea divers and mountain climbers
- It is used as liquid oxygen in rockets when in outer space to support burning of hydrogen
Physical properties of oxygen
- It is colorless
- It is odorless
- It is slightly soluble in water
- It is slightly denser than air
- It supports burning
- It boils at -183 degree Celsius
- It does not burn
Chemical properties of oxygen
1. Respiration
Respiration is the process by living organisms oxidize glucose to produce carbon dioxide, water and energy
2. Combustion
Combustion is the process by which a substance reacts with oxygen to produce an oxide and heat. It is also defined as the burning of substances in oxygen. Alternative term: Burning.
3. Rusting
Rusting is the corrosion of iron. It is an electrochemical process by which iron corrodes in the presence of oxygen, water and an electrolyte
RustChemical name: Hydrated iron (III) oxide. Colour: Reddish – brown
Formation of rust
Iron reacts with oxygen in air in the presence of water to form rust.
Conditions necessary for iron to rust
- Presence of oxygen
- Presence of water or moisture
- Presence of a strong electrolyte such as sodium chloride or sulphuric acid
Prevention of rusting
- Painting A paint coat excludes both air and water from contact with iron
- Oiling Oil or grease may be applied on the surface of iron metal to prevent water and oxygen from contact with iron
- Galvanizing Galvanizing is the coating of iron with zinc metal. Zinc is higher than iron in the reactivity series of metals; so if the surface is scratched, the zinc is oxidized in preference to iron. This is called sacrificial protection.
- Electroplating or alloying Iron can be electroplated or alloyed with non-corrosive elements like nickel, copper, chromium and carbon. The coating of iron with a metal which does not corrode easily protects iron from rusting. An electric current is used in electroplating.