Rates of Chemical Reactions
Rate of chemical reaction is the speed at which the reaction takes place
Measuring the rate of reaction
The rate of reaction can be measured by measuring:
- how quickly a product is obtained
- how quickly a reactant is used up
Example 1
Consider the reaction below
In the reaction above, the rate of reaction can be measured by measuring:
- The volume of carbon dioxide over time
- The decrease in mass of the system due loss of carbon dioxide
Measuring the rate of reaction by measuring the volume of the gas produced
A graduated syringe is used to measure the volume of carbon dioxide gas formed over time
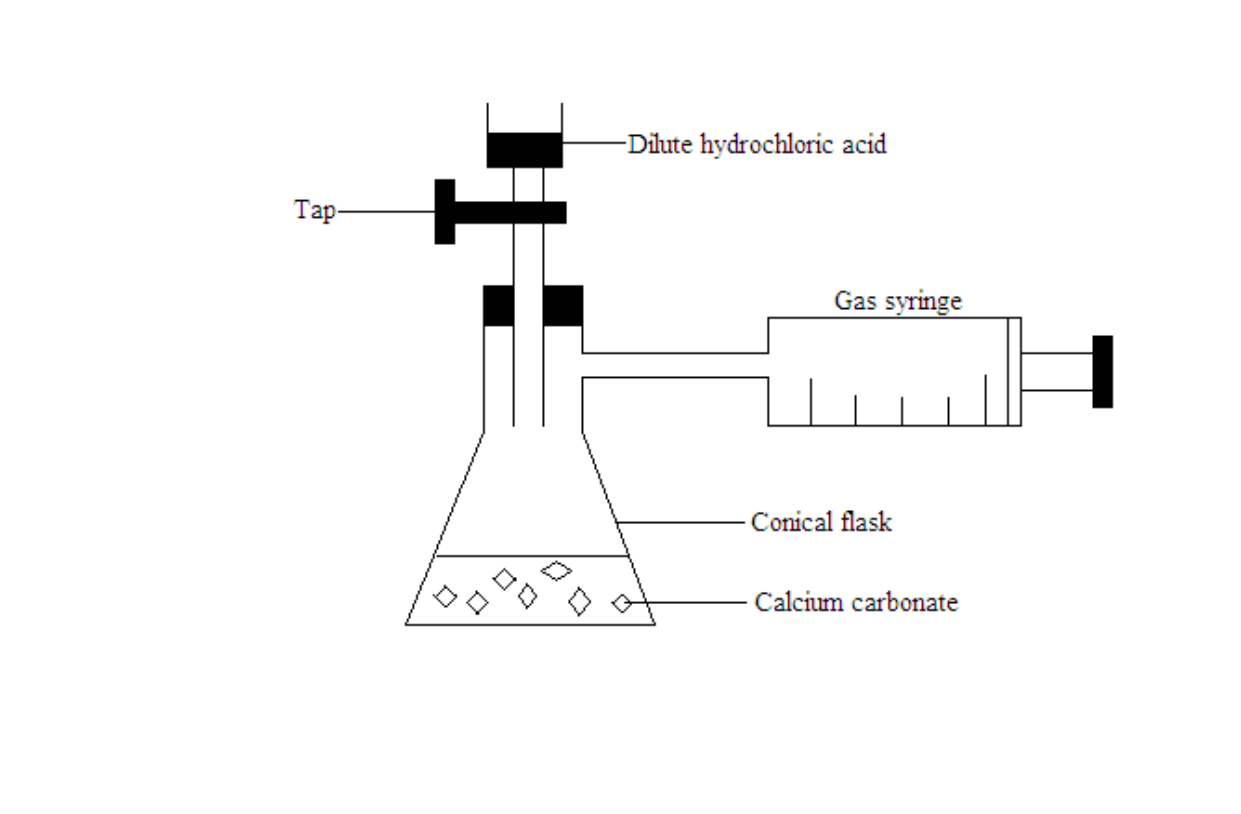
The total volume of carbon dioxide given off at one minute interval is recorded
The graph of total volume of carbon dioxide against time is plotted
The gradient of the graph is calculated. The gradient of the graph is equal to the rate of reaction
Measuring the rate of reaction by measuring the decrease in mass of a system due to loss of product
A mass balance is used to follow the loss in mass of a system
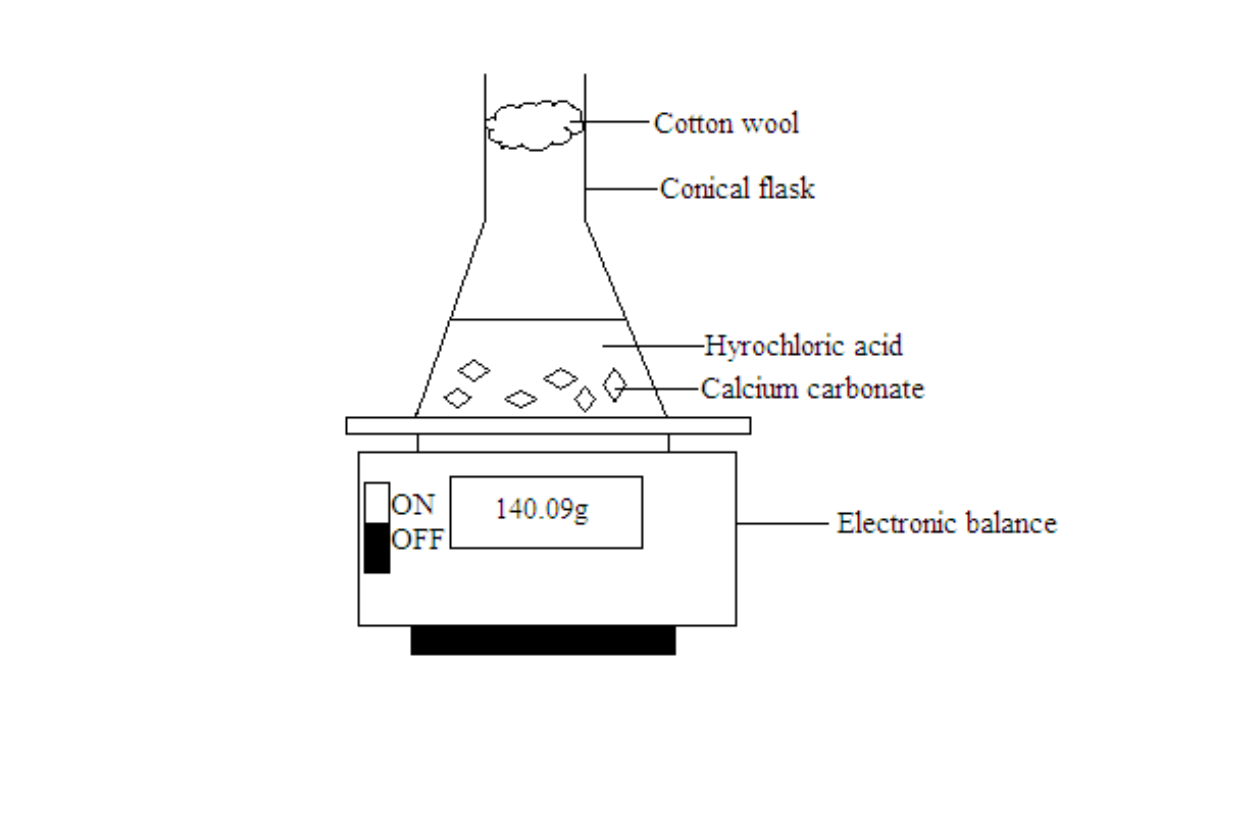
The mass readings will drop over time as the carbon dioxide gas formed escapes. The mass readings are taken at one minute intervals and plotted against time.
The gradient of the graph at various points of the curve will give the rate of reaction. The reaction is fastest at the start because the gradient of the graph is the highest. The value of the gradient decreases with time and finally becomes zero. This means that as the reaction proceeds, the reaction slows down and finally comes to a stop.
Note- The cotton wool is used as a stopper. It will allow the escape of carbon dioxide into the atmosphere and prevent the solution inside the conical flask from splashing out.
- The mass decreases as the reaction proceeds because of the loss and escape of carbon dioxide into the atmosphere.
- When the curve levels off, it means the reaction has stopped.
The collision theory
The collision theory states that: For a reaction to take place, the particles of the reacting substances must move and collide with each other with a certain amount of kinetic energy. The number of collisions taking place per unit time depends on the number of particles If the particles are increased, the number of collisions also increases.
Factors affecting the rate of reaction
- Concentration
- Temperature
- Pressure
- Surface area (size of particles)
- Catalyst
Effects of concentration on the rate of reaction
Concentration refers to the reactants in solution.
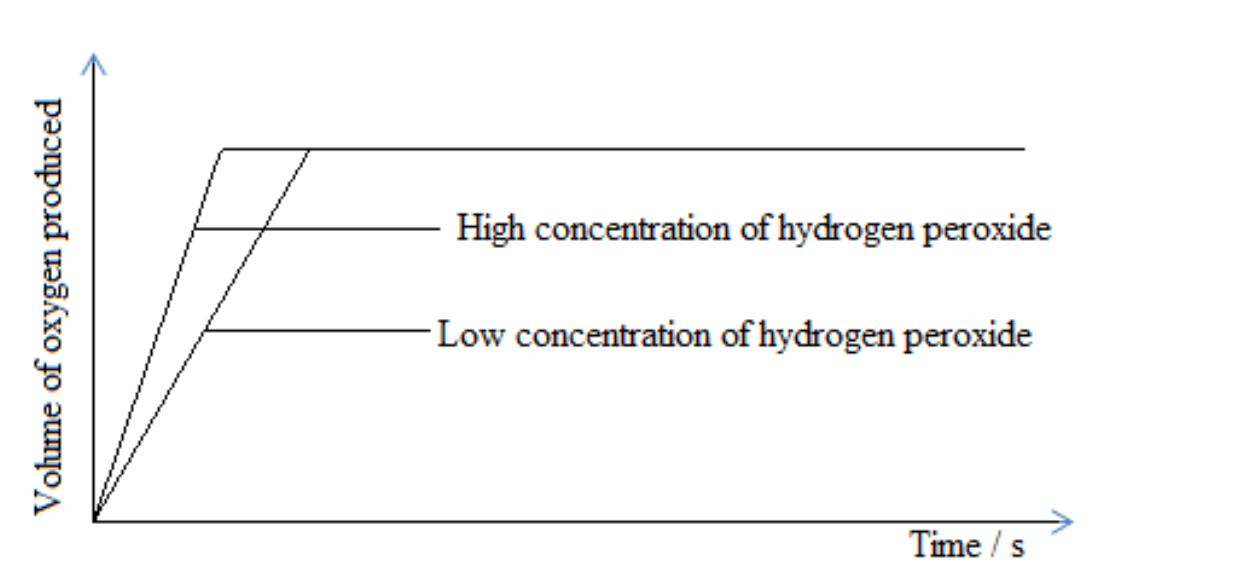
When concentration is increased, the rate of reaction also increases. This is because the number of particles in the solution increases and collides with each other effectively.
On the other hand, when concentration is reduced, the rate of reaction also reduces. This is because the number of particles in the solution reduces and do not collide effectively.
Graphical representation of concentration
Decomposition of hydrogen peroxide
2H 2O2(aq)→2 H2O(l)+O2(g)
Effects of temperature on the rate of reaction
Temperature is the measure of the average kinetic energy of the particles When temperature is increased, the rate of reaction also increases. This because the particle gain kinetic energy and move faster and collide effectively.
On the other, when temperature is reduced, the rate of reaction also reduces. This is because the particles lose kinetic energy and move slower and do not collide effectively.
Note Temperature increases the rate of reactions for endothermic reactions
Effects of pressure on the rate of reaction
Pressure becomes a dominant factor in reactions involving gases When pressure is increased, the rate of reaction also increases. This is because the volume reduces forcing the gas particles closer together and collides effectively
On the other hand, when pressure is reduced, the rate of reaction also reduces. This is because the volume increases and the gas particles are further apart and do not collide effectively.
Effects of surface area (particle size) on the rate of reaction
Particle size usually refers to particles of a solid reactant The rate of reaction is faster when the size of particles is small. This is because a small sized particle has a large surface area.
Note- When a reactant is in solid state, the reaction takes place on the surface of the solid. By breaking up the solid into smaller pieces, the surface area is increased giving a greater area for collisions to occur. This results in an increase of the rate of reaction.
- This explains why mixtures of saw dust, fine products of flour mills and combustion of gases can cause an explosion due to the large surface area.
Effects of a catalyst on the rate of reaction
A catalyst is a chemical substance which alters the rate of reaction but remains chemically unchanged at the end of the reaction.
A catalyst usually speeds up the reaction by lowering the activation energy of the reaction.
Activation energy
Activation energy is the minimum energy required to start a reaction. As a result, a catalyst allows a reaction to go by a different pathway with lower activation energy allowing more collisions for a successful reaction.
Activation energy is usually the energy barrier because if any collision is not energetic enough, the reaction will be futile Some catalysts slow down the reactions; these are called inhibitors (negative catalysts)
Characteristics of a catalyst
- It catalyzes both the forward and reverse reaction
- It undergoes physical change
- It remains chemically unchanged at the end of the reaction
- It is only needed in very small amounts
- It is poisoned or rendered useless in the presence of impurities
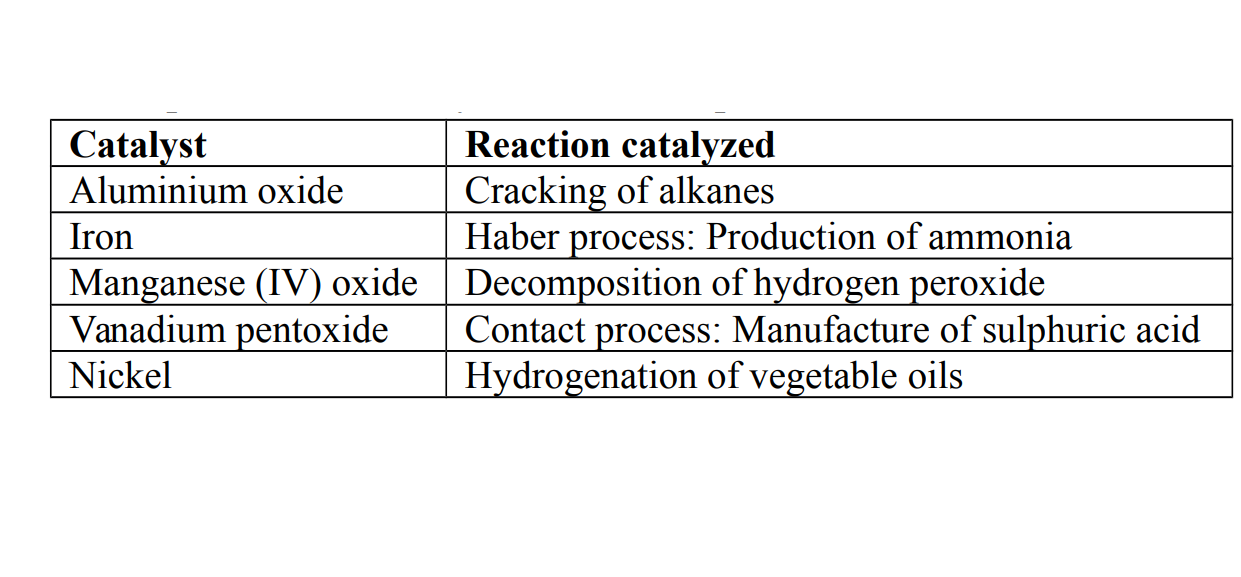
Examples of some catalysts used for important reactions
Chemical equilibrium
Equilibrium is the point where the rate of forward reaction is equal to the rate of reverse reaction.
Reversible reactions
A reversible reaction is a reaction that proceeds in the forward and backward directions It follows that if the reaction is exothermic, then the reverse reaction will be endothermic and vice versa . Symbol for reversible reactions: ⇌
Example: A + B ⇌ C + DThe double headed arrow shows that the reaction is a reverse reaction
Characteristics of equilibrium reactions
- Rate of forward reaction is equal to rate of backward reaction
- Concentration of reactants and products remain constant at equilibrium
- An equilibrium can only be established in a closed system
- There is no loss or gain of external materials by the reacting system
Le Chartelier’s Principle
The principle states that: If an equilibrium is disturbed by a changing the conditions the reaction moves to counteract the change
Factors that affect the position of equilibrium
Temperature changes
Temperature variations changes the position of the equilibrium of either endothermic or exothermic reactions.
An increase in temperature favors the forward reaction of endothermic reaction while a decrease in temperature will shift the equilibrium backwards
Example: NH4Cl(s) ⇌ NH3(g) + HCl(g)In thermal decomposition of ammonium chloride, temperature increase cause equilibrium to shift to the right producing ammonia and hydrogen chloride. On the other hand, when temperature is reduced, equilibrium shifts to the left and the backward reaction is favored producing ammonium chloride
Concentration changes
Generally, an increase in concentration of the reactants of an equilibrium reaction favors the forward reaction. This is because the equilibrium will adjust itself so as to offset the effect of adding more reactants. On the other hand, if the concentration of products is increased, the backward reaction will be favored so that the reactants are produced to restore the balance
Pressure changes
In gaseous systems, an equilibrium reaction is followed by volume change. Therefore, the equilibrium is affected by change in pressure.
Example: N2(g) + 3N2(g) ⇌ 2NH3(g)In the production of ammonia, there is a general decrease in volume and a consequent increase in pressure. Therefore, an increase in pressure will make the equilibrium shift towards the reduction of volume. This means the forward reaction is favored and more ammonia is produced. Consequently, a decrease in pressure leads to the production of nitrogen and hydrogen; it favors the backward reaction
Generally, gaseous reactions that lead to reduction in volume are favored by high pressure.
Energy changes
Energetics refers to the energy changes that characterize chemical reactions. Alternative term: Energetics
Terms related to energy changes
Enthalpy
This is the total energy in one mole of a substance. It is also the heat content of a reacting system. It depends on the physical state of the compound and varies from compound to compound
Symbol: H
Enthalpy change of reaction
This is the difference between the enthalpy of the products and the enthalpy of the reactants
Symbol: ∆H (delta H)
Units: Kilojoules per mole, Kj/mol
Endothermic reaction
This is a reaction which absorbs heat energy from the surroundings
Endothermic reaction is a bond breaking process
Examples of endothermic reactions (a) Photosynthesis (b) Photography (c) Dissolving processes such as dissolving ammonium nitrate or potassium nitrate in water.
In endothermic reaction:
- Energy is absorbed from the surroundings
- Reactants have less energy than products
- ∆H is positive
- Temperature of the system fall
Exothermic reaction
This is a reaction in which energy is released to the surroundings
Examples of exothermic reactions (a) Combustion (b) Tissue respiration (c) Dissolving sodium hydroxide crystals in water
Exothermic reaction is a bond forming process
In exothermic reactions:
- Energy is released to the surroundings
- Reactants have more energy than products
- ∆H is negative
- Temperature of the system increases
- Bonds formed are relatively stronger than bonds broken
Bond energy
This is the amount of energy required to either break the bond or energy released when one mole of bond is formed. Alternative term: Bond enthalpy
Calculating enthalpy change using bond energies
∆H = ∑ bond energies of reactants – ∑ bond energies of products
Example 1
Calculate the enthalpy change for the reaction between hydrogen and chlorine gas given the following bond energies.
Solution
∆H = ∑ bond energies of reactants – ∑ bond energies of products
= (436 + 243) – (2 x 431)
= 679 – 862
= -183Kj/mol
Electrochemistry
Electrolysis
Electrolysis is the decomposition of an electrolyte by using an electric current
Electrolyte
An electrolyte is a substance which conduct electricity in fused (molten) or in solution and is thereby decomposed
Examples of electrolytes
- Aqueous sulphuric acid
- Aqueous hydrochloric acid
- Nitric acid
- Aqueous sodium chloride
- Aqueous sodium hydroxide
- Aqueous carbonic acid
- Aqueous ethanoic acid
Strong electrolyte
It is a substance which ionizes completely and produces a lot of ions in solution which are able to carry out an electric current rapidly.
Examples of strong electrolytes
- Aqueous sodium hydroxide
- Aqueous sodium chloride
- Aqueous copper (II) sulphate
- Aqueous hydrochloric acid
- Aqueous sulphuric acid
- Aqueous nitric acid
Weak electrolyte
It is a substance which ionizes partially and it conducts electric current only slightly and therefore undergoes slight decomposition.
Examples of weak electrolytes
- Carbonic acid
- Organic acids e.g. ethanoic acid
Non electrolyte
It is a substance which does not conduct electricity I fused or in solution state
Examples of non-electrolytes
- Sugar solution
- Ethanol
- Petrol
- Benzene
- Tetra chloromethane
They do not conduct electricity because they exist only in form of molecules and not capable of ionization
Conductor
It is a solid substance that allows electricity to pass through without decomposing e.g. all metals
Non conductor
It is a solid that does not conduct electricity e.g. plastics, wood and glass. Alternative term: Insulator
Electrodes
They are conductors that allows electricity in and out of an electrolyte
Anode
It is a positively charged electrode. It is an electrode connected to the positive terminal of the power supply.
Cathode
It is a negatively charged electrode. It is an electrode connected to the negative terminal of the power supply.
Cations
They are positively charged ions
Anions
They are negatively charged ions
The ionic theory
The ionic theory states that: The electrolytes contain ions and when no current is passing, the ions wander about randomly in the solution. If the electric circuit is closed, the cathode immediately becomes negatively charged and the anode becomes positively charged. The anode attracts negatively charged ions while the cathode attracts the positively charged ions.
The selective order of discharge of ions
The discharge of ions varies from one electrolyte set up to another. When two or more ions of similar charge are present under similar conditions in a solution.
The ion selected for discharge of competing ions depends on the following factors:
- The position of ions in the electrochemical series
- The concentration of ions in solution
- The nature of electrodes
Position of ions or radicals in the electrochemical series
Ions are arranged in the order of decreasing order of stability and amount of energy they require for them to get discharged from an aqueous solution when an electric current is made to flow through the solution.
The arrangement is called electrochemical series. Note that the electrochemical series is slightly different from the reactivity series of metals.
Concentration of ions in aqueous solution
Concentration has no effect on the selective discharge of metal cations. However, the concentration of anions is the principle factor that determines which anions will be liberated regardless of their position in the electrochemical series.
The anion with the highest concentration is selectively discharged from the solution in preference to the one whose concentration is lower regardless of their position in the electrochemical series. For example, in the electrolysis of concentrated sodium chloride, both OH- and Cl- are present.
Nature of electrodes
Insert or unreactive electrodes such as platinum and graphite (Carbon) have no effect on the selective discharge of competing ions on their surfaces. However, active electrodes such as copper, mercury and most metals have an effect on the preferential discharge of competing ions.
An active electrode selectively discharges more stable ions in preference to less stable ions. For example, in the electrolysis of sodium chloride solution using inert electrodes, H+ are discharged at the cathode in preference to Na+ .
Industrial application of electrolysis
- Electrolysis is used in extraction of very reactive metals such as potassium, sodium, magnesium, calcium and aluminium
- Electrolysis is used in the refinery and purification of metals such as copper and zinc.
- Electrolysis is used in the electroplating metals. Electroplating is the art of covering the surface of a metal with a thin adherent metal coating by means of electrolysis. Electroplating is done to protect the surface of the base metal against corrosion or for a purely decorated effect. Metals that may be used for electroplating include nickel, silver, gold, chromium, zinc, tin
Faraday’s laws of electrolysis
Faraday’s first law of electrolysis
The law states that: The mass of a substance produced at the electrode during electrolysis is directly proportional to the quantity of electricity passing through the electrolyte.
m ∞ Q
m ∞ It
Q = It
m = mass[g]
I = current [A]
t = time [s]
Q = charge (quantity of electricity) [Coulombs, C]
1. A current of 0.4A flows for about 1500 seconds. Calculate the quantity of electricity.
Solution
Q = It
Q = 0.4A x 1500s
Q = 675C
Faraday’s second law of electrolysis
The law states that: When the same quantity of electricity is passed through different electrolytes the number of moles of the element deposited is inversely proportional to the charges on the ions of the element.
1 Faraday = 1 mole of electrons = 96500C
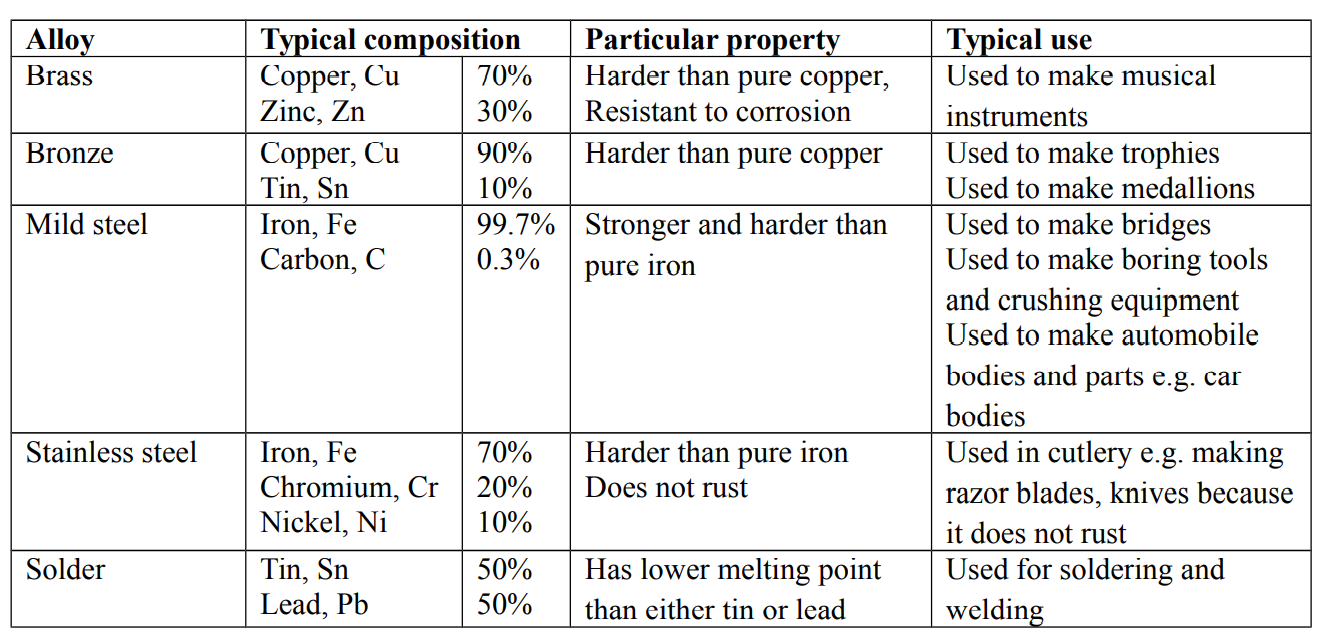
Alloys
An alloy is a mixture of two or more metals or a metal with a non-metal
The combination of alloys is physical
Alloys are harder than the metals from which they are made
Alloying a metal is one way of increasing its strength
Preparation of alloys
The mixture is usually heated under controlled temperature. The molten mixture is then allowed to cool and solidify
Advantages of alloys
- They are flexible in use
- They usually have improved appearance
- They are durable and reliable
- They have increased resistance to corrosion
Examples of alloys
Metals
General physical properties of metals
- Metals are good conductors of heat and electricity.
- Metals are malleable i.e. they can be hammered into thin sheets
- Metals are ductile i.e. they can be drawn into long wires
- Metals are sonorous i.e. they produce sound when hammered
- Metals are lustrous i.e. they have a silver luster surface when freshly cut
- Metals are solids at room temperature and pressure except for mercury which is a liquid at room temperature and pressure
- Metals have high melting and boiling points
The reactivity series
Reactivity is a list of metals with the most reactive metal at the top and the least reactive metal at the bottom. Alternative term: Activity series.
The order of reactivity can be determined by the reaction of the metal with water or steam and acids. In both types of reaction, if a reaction takes place, hydrogen gas is formed.
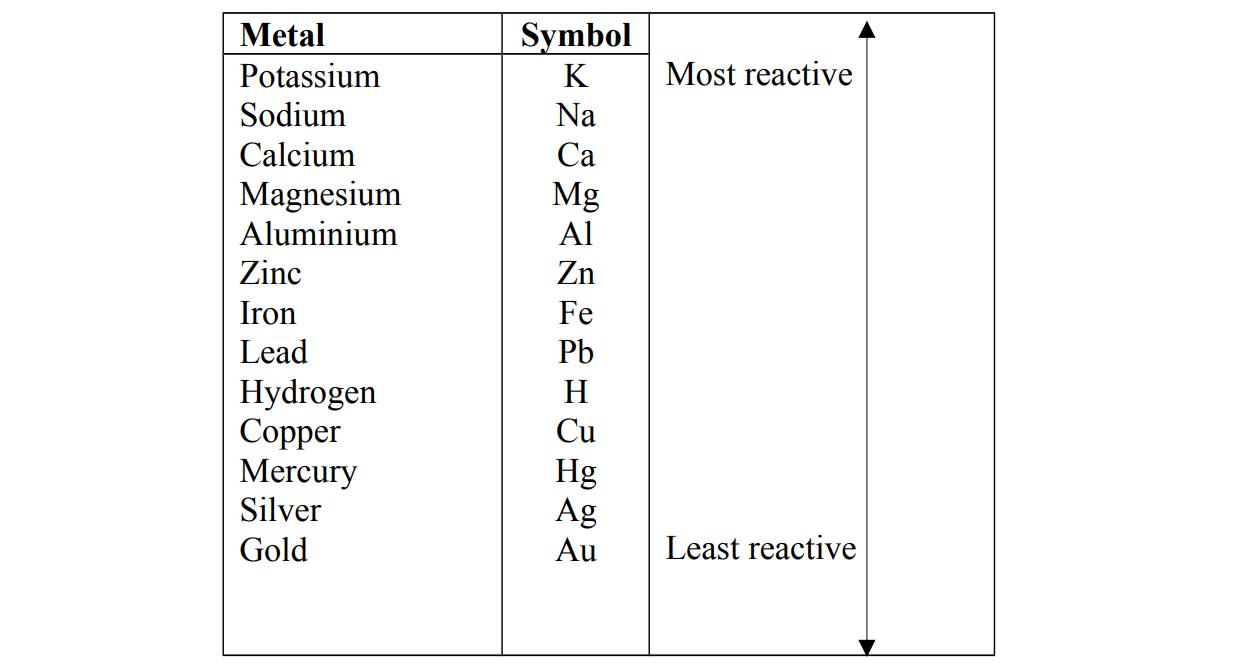
The reactivity series is related to the tendency of metals to form positive ions Very reactive metals lose their valence electrons easily to form cations Metals at the top of series lose the electrons more easily and form ions rapidly and are called electropositive.
Metals at the bottom of the series lose electrons with difficulty and do not readily form ions and are said to be less electropositive.
Recycling of metals
Metals are finite resources. It is essential that we recycle metals that are still useful to us.
Advantages or reasons of recycling metals
- Better conservation of natural resources, so that reserves last longer. The demand for metal ores will decrease once scrap metal is identified as a viable source of raw material.
- With recycling, less mining will take place. There will be less air and water pollution caused by mining process
- More effective waste disposal as scrap metal is recovered. Less landfill space will be needed. This will also solve the problem of litter accumulation
Recycling is sometimes not feasible because of the costs involved. Transportation, sorting through waste and cleaning the scrap metal etc. may cost more than extracting the metal from ores. This is true for some cheaper metals.
Extraction of metals
Most metals occur in the earth’s crust as ores. An ore is a compound from which a metal can be extracted.
Methods of extraction
The method of extraction of a metal from the ore depends on its position in the reactivity series There are three main methods used to extract metals from their ores
1. Electrolysis
2. Reduction
3. Thermal decomposition
Stability of compounds
Compounds of very reactive metals like potassium, sodium, calcium, magnesium and aluminium cannot be decomposed by heating or reduction method using hydrogen, carbon or carbon monoxide as reducing agents.
Effect of heat on carbonates
(a) Carbonates of group I elements like potassium, sodium, rubidium are extremely stable and hence cannot be decomposed by heat
(b) Carbonates of group II elements like calcium, magnesium, barium and transition elements like zinc, iron, lead, copper etc. are only decomposed to oxides and carbon dioxide when heated. No further decomposition is possible after this. The oxides are extremely stable and can only be reduced by electrolysis.
Aluminium
Aluminium is the most abundant metal in the earth’s crust
BauxiteChemical name: Aluminium oxide
Formula: Al2O3
The oxide is very stable and hence cannot be decomposed by heat or reduction with carbon
Extraction of aluminium
Aluminium is extracted by electrolysis of molten bauxite, Al2O3 in the electrolytic cell. Extraction of aluminium from bauxite is carried out in cell graphite (carbon) electrodes.
Bauxite is dissolved in cryolite to lower its lower melting point. Aluminium is formed at the cathode and settles at the bottom in molten form Aluminium is tapped out by opening an outlet when it has accumulated
The oxygen gas immediately reacts with the graphite electrodes to form carbon dioxide gas since they are made of carbon. For this reason, the anodes are eaten away and hence they are replaced at regular intervals.
Three main stages in the extraction of aluminium from bauxite
Bauxite is impure aluminium oxide,
- Bauxite is purified
- Pure aluminium oxide is dissolved in molten cryolite,
- Electrolysis is performed in the cell
The apparent unreactivity of aluminium
Despite being high in the reactivity series, aluminium does not easily react with water and oxidizing acids like nitric acid and sulphuric acid.
Aluminium forms aluminium oxide in the presence of air. This is because shortly after being extracted out, a thin protective layer of aluminium oxide forms on its surface.
This oxide is insoluble and resistant to corrosion. So it forms a protective coating for aluminium.
Uses of aluminium
- It is used in overhead electrical cables because it is a good conductor of electricity
- It is used in making cooking utensils like sauce pans and kettles because it is a good conductor of heat and it is resistant to heat
- It is used in making food wrappers and drink cans due to its resistance to corrosion and is non-toxic
- It used in the manufacture of aeroplanes because it is lighter (low density) and has high strength. It is cheaper and therefore preferred than copper
- It is used in making light aluminium roofing sheets
- It is used in making alloys e.g. duralumin
- It is used in making aluminium paints. The powdered metal is used with oil
Copper
MalachiteChemical name: Copper (II) carbonate
Copper pyritesChemical name: Copper (I) sulphide
Extraction of copper
Copper is extracted from copper (I) sulphide by thermal decomposition. This is usually done in the presence of oxygen. The copper (I) sulphide is reduced to copper by heating in a controlled supply of air. The impure copper is called blister copper.
Purification of copper
The copper formed is impure. Silver, gold and cobalt are usually present as impurities. It is purified by electrolysis
The electrolyte is an acidified solution of an electrolyte containing the metal ion The impure copper anode loses mass as copper ions are formed.
At the anode pure copper from pure anode goes into solution, reducing the size of the anode
Impurities called anode sludge fall to the bottom of the cell. The anode sludge may contain valuable metals such as silver and gold.
At the anode copper ions accept two electrons each and are deposited as pure copper metal on the electrode which increases in size.
Uses of copper
- it is used in making electric cables because it is a good conductor of electricity
- it is used in making alloys e.g. bronze and brass
- it is used in making coils
- it is used in making ornaments in jewelry industries e.g. coins, necklaces and rings
- it is used in making cooking utensils and boilers since it is a good conductor of heat
Zinc
Zinc blendChemical name: Zinc sulphide
CalamineChemical name: Zinc carbonate
Extraction of zinc
Zinc blend is heated in air to form zinc oxide and sulphur dioxide
Zinc oxide is then reduced to zinc metal by carbon monoxide
Uses of Zinc
- It is used in making alloys e.g. brass which is an alloy of zinc and copper
- It is used to galvanize iron to prevent rusting
- It is used in making roofing sheets
- It is used in the preparation of dry cell batteries
Iron
HeamatiteChemical name: Iron (III) oxide
MagnetiteChemical name: Tri iron tetra oxide
SideriteChemical name: Iron (II) carbonate
Extraction of iron
Iron is extracted from Heamatite by the reduction method in the blast furnace
The blast furnace
The blast furnace is tower of about 30 – 40 meters high. It is made of steel, and lined with fire proof bricks with a high melting point.
To extract iron, three substances called charge (raw materials) are mixed together. These are:
Iron ore: The chief ore is heamatite, Fe2O3
Lime stone: This is calcium carbonate, CaCO3
Coke: This is pure carbon, C
The charged is crushed and placed into the top of the blast furnace. It is then roasted in air.
Chemical reactions in the blast furnace
- Coke reacts with oxygen in hot air to form carbon dioxide. This is an oxidation process. The reaction rises the temperature in the blast furnace.
- Carbon dioxide rising up reacts with more coke to form carbon monoxide Carbon (coke) is a reducing agent because it reduces carbon dioxide to carbon monoxide
- Carbon monoxide react with iron oxide to form liquid iron and carbon dioxide Carbon monoxide gas is a reducing agent because it reduces the iron oxide to iron
- Limestone decomposes to calcium oxide and carbon dioxide. The purpose of limestone is to act as a flux
- Calcium oxide reacts with silicon dioxide (sand) to form calcium silicate or slag. The slag runs down the furnace and floats on the iron. This prevents the molten iron from being oxidized by the incoming oxygen. Slag is tapped off. Calcium oxide is used to remove impurities such as silicon dioxide
Uses of slag
- It is used in the manufacture of cement
- It is used for making roads
Note: The raw iron obtained in this process is called cast iron or pig iron. It contains impurities. The purest form of iron is called wrought iron.