Bonding of Atoms
Bonding is chemical combination of two or more atoms. Compounds and molecules result from chemical bonding Only outer most shell electrons take part in bonding. Atoms are held together by the forces of attraction or bonds. A bond is a force of attraction between atoms.
A compound is made of atoms of different elements, bonded together. The compound is described by a formula, made from the symbols of the atoms in it. (The plural of formula is formulae.)
Molecules Is a smallest particle of a substance that has both the physical and chemical properties. It is made up of one or more atoms that come together,
Why do atoms form bonds?
Atoms form bonds in order to be stable. Atoms react with one another in order to acquire full outer most shells like those of noble gases.
An ion is a charged particle
There are two types of ions namely cations and anions. A cation is a positively charged particle meaning It has more protons than electrons while An anion is a negatively charged particle, An anion has more electrons than protons.
Structure of noble gases
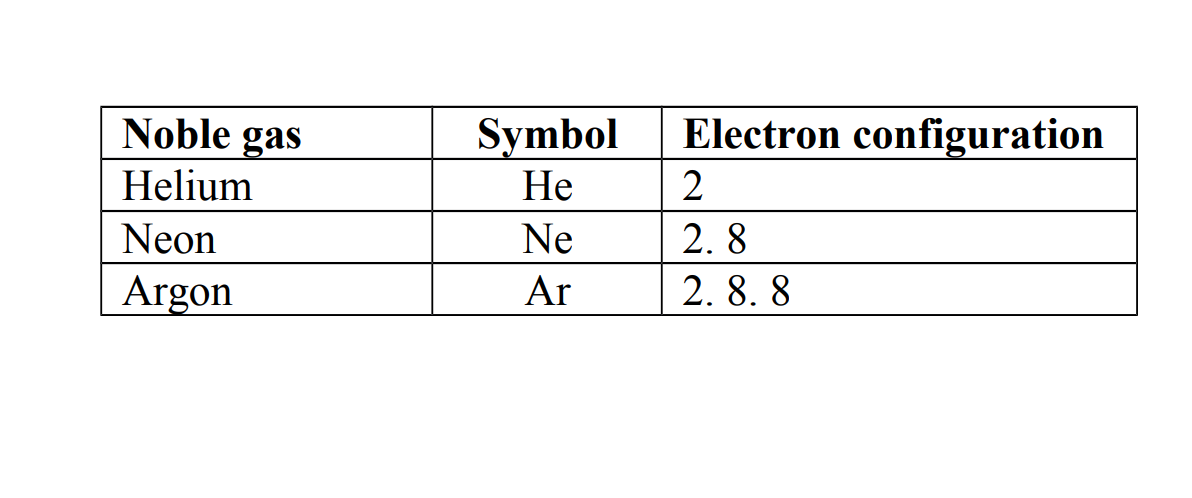
Noble gases are atoms that have eight electrons in the outer most shells except helium which has only two electrons.
Other noble gases which have eight electrons in their outer most shells obey an octet rule.
Examples of noble gases
Types of bonding
There are three types bonding
- Ionic bonding
- Covalent bonding
- Metallic bonding
Ionic bonding
Ionic bonding involves the transfer of electrons from a metal to a non-metal A metal loses electrons while a non-metal gains electrons. Alternative term: Electrovalent bonding.
An ionic bond is the force of attraction between oppositely charged ions.
Electrovalency
Electrovalency is the number of electrons lost or gained by an atom.
Example of ionic bond
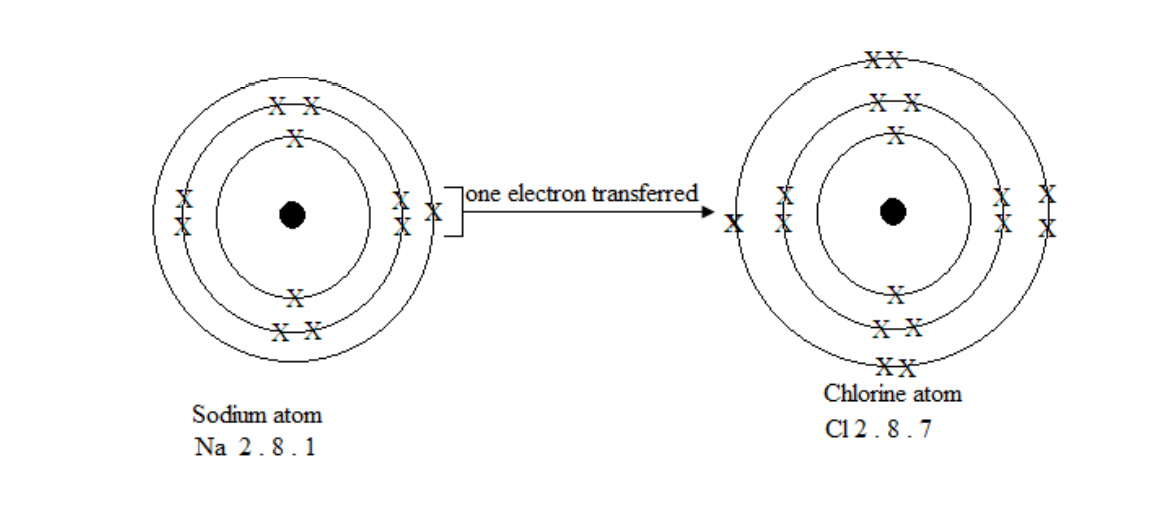
Formation of an ionic bond
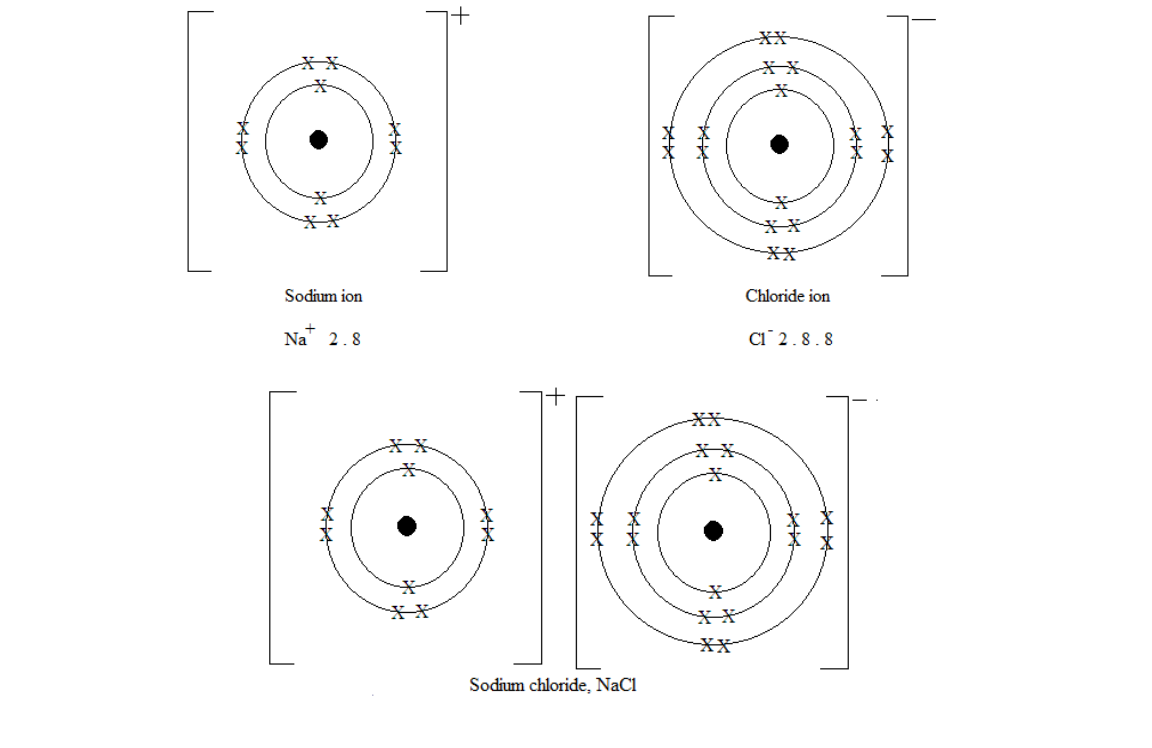
Formation of sodium chloride
chloride and the ionic bond is formed between the oppositely charged ions.
Na + Cl = NaCl
Formation of sodium chloride NaCl
Covalent bonding
Covalent bonding involves the sharing of the outer most electrons between non-metal atoms. Alternative term: Molecular bonding.
A covalent bond consists of a shared pair of electrons and it is formed between non-metals which share one or pairs of electrons. Atoms are held by the attraction between their positive nuclei and the shared electrons.
Covalency is the number of electrons an atom shares with another atom.
Covalent compounds
Covalent compounds are usually molecules. A molecule the smallest particle of an element or compound which exists independently, that is in a free state. Alternative term: Molecular compounds.
They are formed when non-metals combine by sharing electrons. As a result of sharing electrons, each non-metal acquires a completely filled outer most shell.
Examples of covalent compounds
Formation of Hydrogen molecule
Hydrogen
H + H →
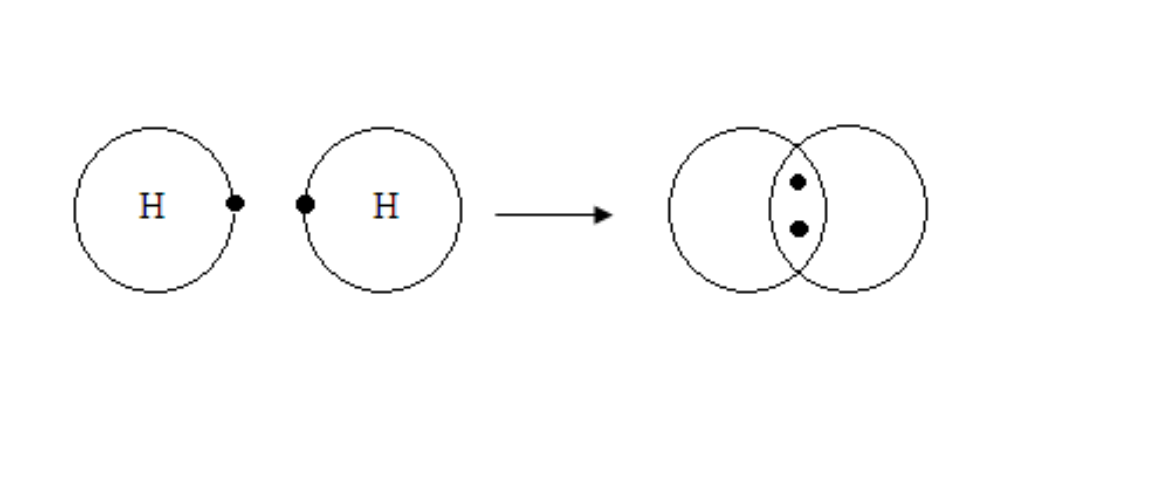
Formation of water molecule
Water
H – O – H
H + O + H →
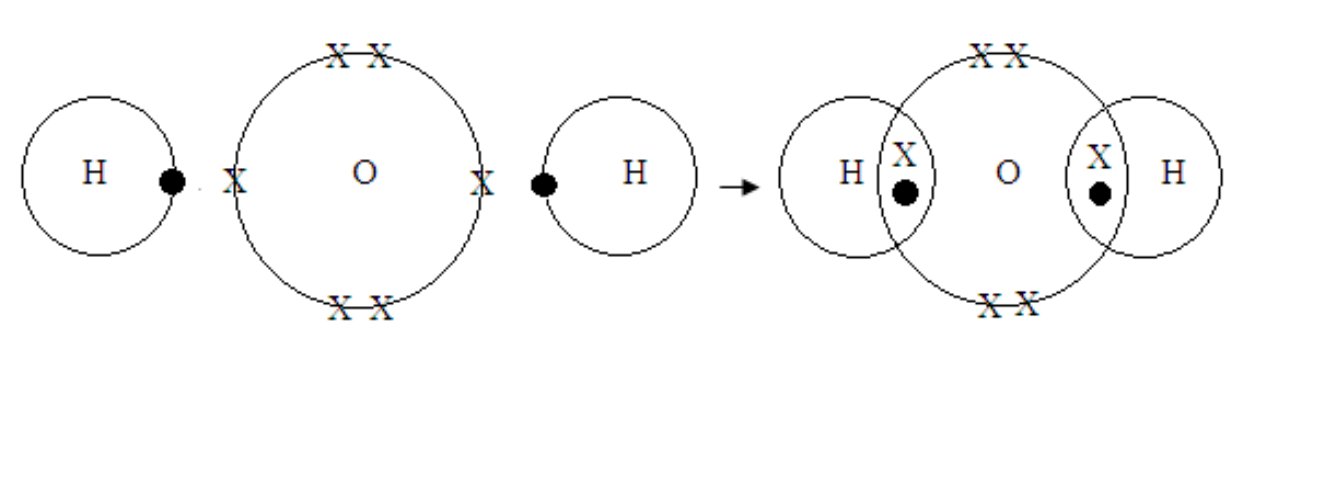
Metallic bonding
Metallic bonding is the attraction between the positively charged metal ions and the free electrons in a metallic lattice.
It involves the sea of electrons around positively charged particles inside a metal structure.
The electrons are free to move anywhere in the metallic lattice. The electrons are said to be delocalized.
Sodium atoms, for example, lose a single electron from the outer most shell. When a large number of sodium atoms lose these electrons, the result is many free electrons.
Sodium metal lattice
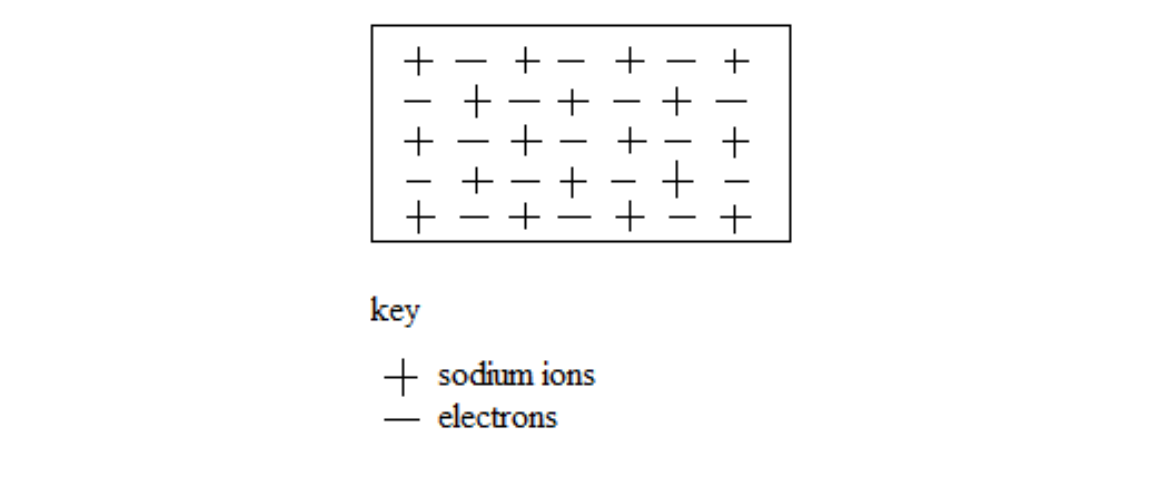
Valency – Combining power
Valency is the number of electrons lost or gained or shared by an atom of the element to attain a stable structure
Valencies of some elements
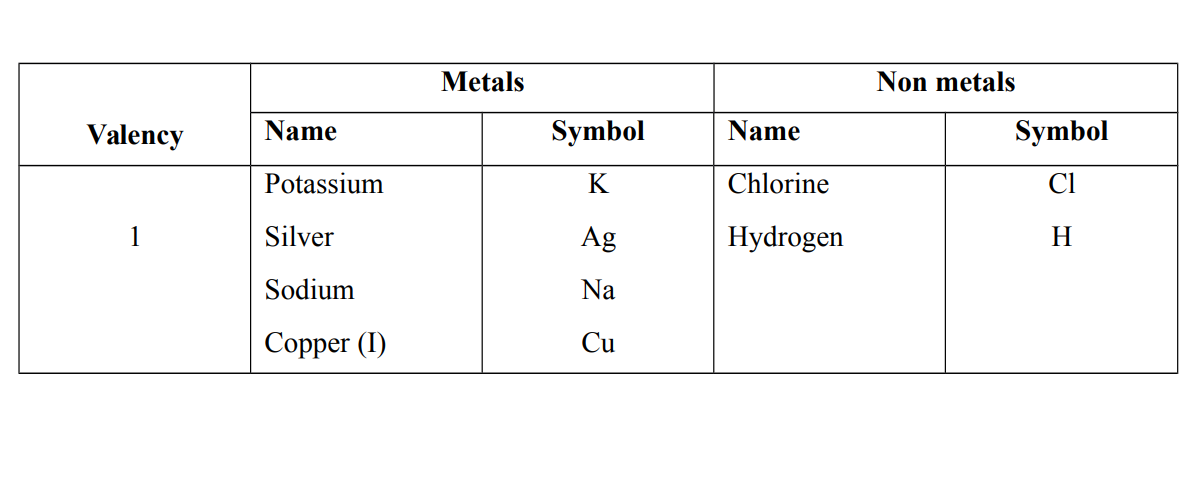
Note that the valency is the number of electrons on the outer shell or last shell e.g Sodium Na = 2. 8. 1
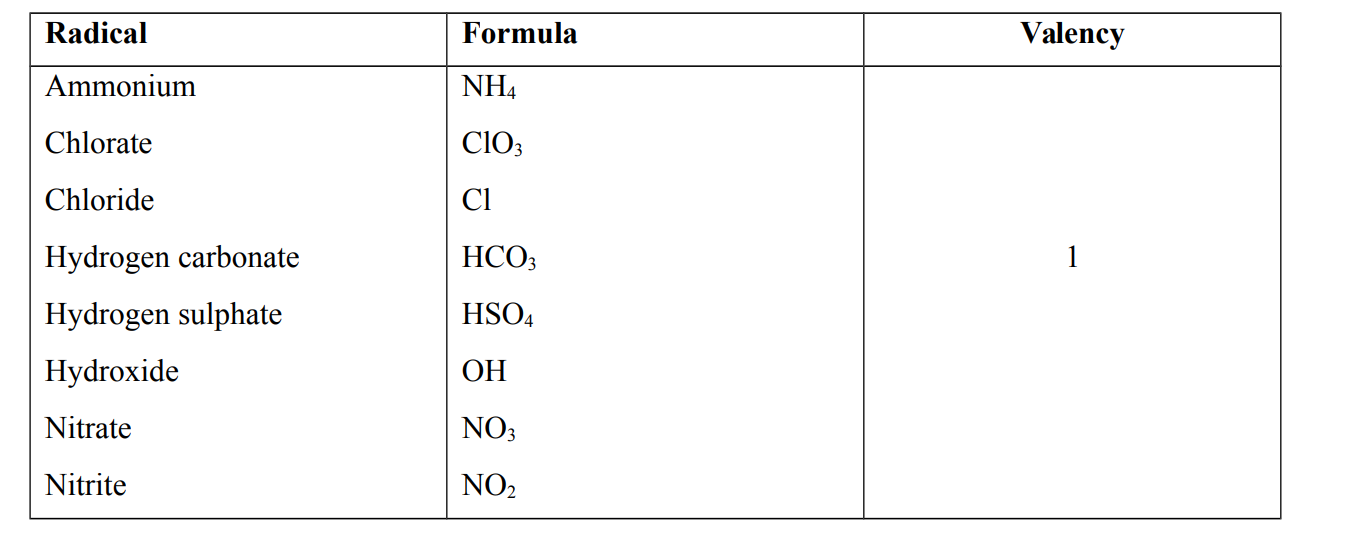
Radical
A radical is a group of atoms which is present in several compounds but incapable of independent existence. e.g Ammonium, Chloride, Chlorate, Carbonate , Hydrogen carbonate, Hydroxide and Nitrate
Radicals and their Valencies