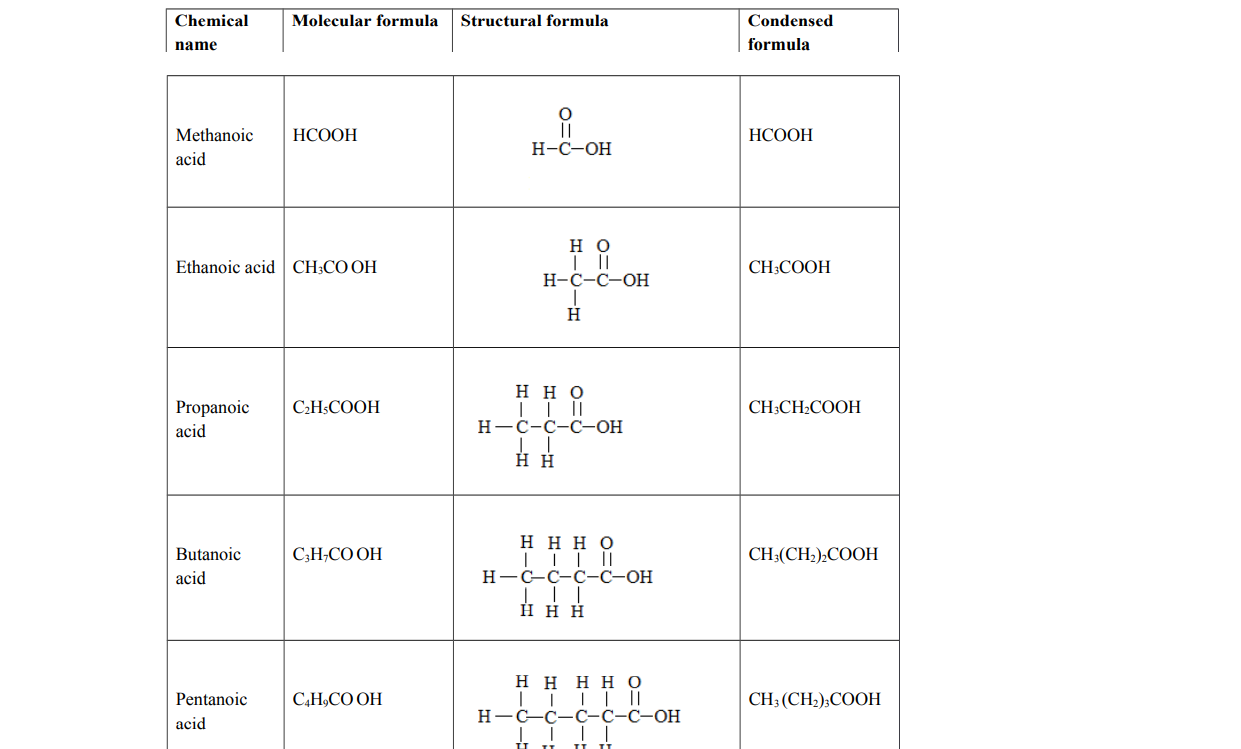
Carboxylic acids
Alternative term: Alkanoics
General molecular formula: Cn H2n+1COOH where n = 0, 1, 2, 3
Functional group: Carboxyl group, ─ COOH. They end with anoic acid.
Examples of carboxylic acids
Preparation of ethanoic acid
(a) Oxidation of ethanol
(I) Ethanoic acid can be prepared by the oxidation of ethanol by bacteria in atmospheric air.
C2 H5OH + O2 → CH3COOH + H2O(II) Ethanoic acid can also be prepared by the oxidation of ethanol using an oxidizing agent e.g. Acidified potassium dichromate(VI)
C2 H5OH + 2[O] → CH3COOH + H2OThe oxygen is from the oxidizing agent.
The orange acidified potassium dichromate (VI) solution turns green in this reaction.
Physical properties of carboxylic acids
- They turn blue litmus paper red
- They have PH values less than 7
- They have a sour taste
Chemical properties of carboxylic acids
1. They react with reactive metals to form a salt and hydrogen gas
e.g 2Na + 2CH3COOH → 2CH3COONa + H2
2. They react with bases to form a salt and water only
NaOH + CH3COOH → CH3COONa + H2 O
3. They react with carbonates and hydrogen carbonates to form a salt, water and carbon dioxide
Na HCO3 + CH3COOH → CH3COONa + H2 O + CO2
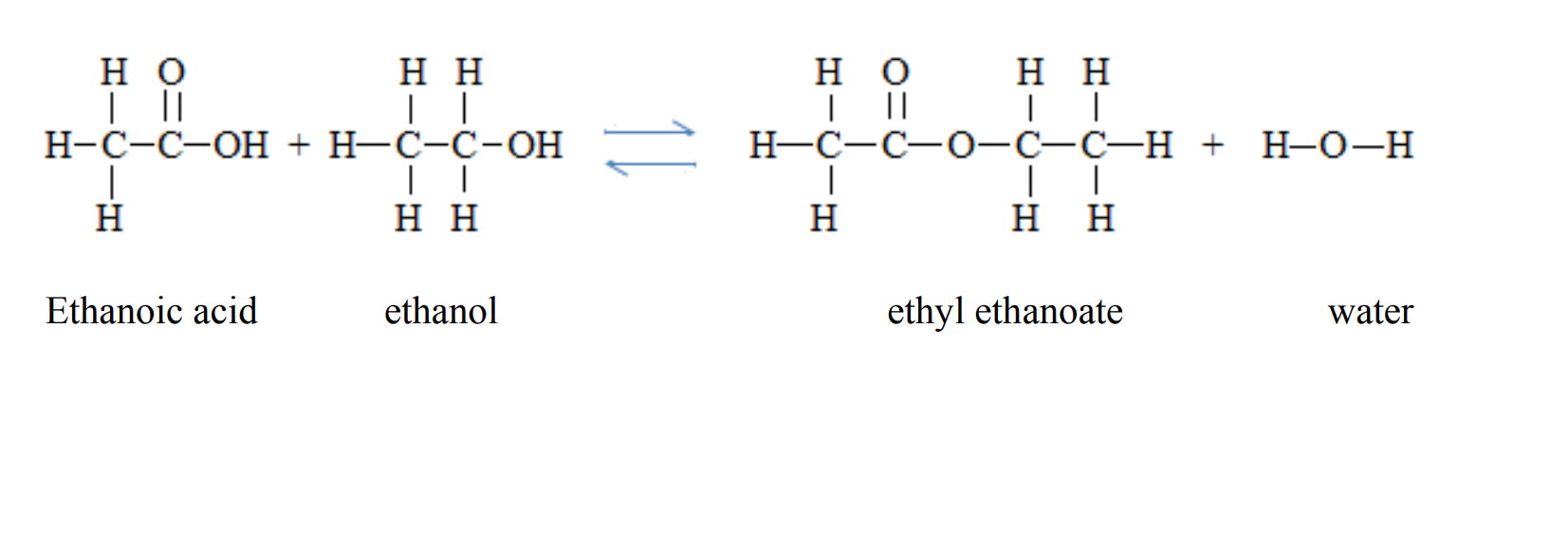
4. They react with alcohols to form esters and the process is called esterification. Esterification is a reaction between a carboxylic acid and an alcohol to form an ester in the presence of sulphuric acid. Esters are sweet smelling compounds.
Ethanoic acid can react with ethanol to form an ester called ethyl ethanoate and water. In this reaction, ethanoic acid loses the –OH group while ethanol loses the –H group to form water. The remaining sections of the molecules join together to form the ester.
CH3COOH + C2 H5OH ⇌ CH3COOC2 H5 + H2O
Conditions for esterification
Temperature: 180 degree Celsius
Catalyst: Sulphuric acid
Note The name of the ester follows the order: alcohol, then acid. For example, if methanol reacts with propanoic acid, the ester formed will be called methyl propanoate.
Reflux condenser: It is held vertically to prevent the escape of any unchanged ethanol. Ethanol has a low boiling point and vaporizes easily. When the ethanol vapour comes into contact with the cold surface of the condenser, it will liquefy and return to the flask.
Esterification is reversible ⇌
The back ward reaction is called hydrolysis
Water can react with ethyl ethanoate to form ethanoic acid and ethanol. To prevent hydrolysis, sulphuric acid is added to remove (absorb) water.
Special property of esters
1. Esters have sweet smells
Uses of esters
- They are used in perfumes due to sweet fruit smells
- They are used in food and drink flavouring and preservation
Similarities between esterification and neutralization
- Both reactions produce water
- Both reactions are exothermic
Differences between esterification and neutralization
- Esterification is reversible while neutralization is not reversible
- Esterification produces an ester while neutralization produces a salt
- Esterification is slower while neutralization is faster
- Esterification involves a carboxylic acid (organic acid) and an alcohol while neutralization involves any acid (organic acid or mineral acid) and a base.