Atomic Physics
Nucleus
Composition of atom
- An atom consists of proton , neutron and electron .
- A Proton is positivery charged
- An electron is negatively charged.
- A neutron is neutral.
- Protons and neutrons are bound together in the nucleus .
- Protons and neutrons is called the nucleon.
- Electrons move round the nucleus in different orbits called shells .
- Electrons and protons carry equal numbers in an atom.
- If atom is neutral, the number of electrons is the same as the number of protons.
Atomic number and Mass number
- The number of protons in an atom is called the atomic number or proton number (Z).
- The total number of nucleons in a nucleus is called the mass number or nucleon number (A).
- If the number of neutrons in the nucleus is N,
Formula for Mass number
A: Mass number
Z: Atomic number
N: Number of neutrons
An element of chemical symbol X with a mass number A and an atomic number Z is expressed,
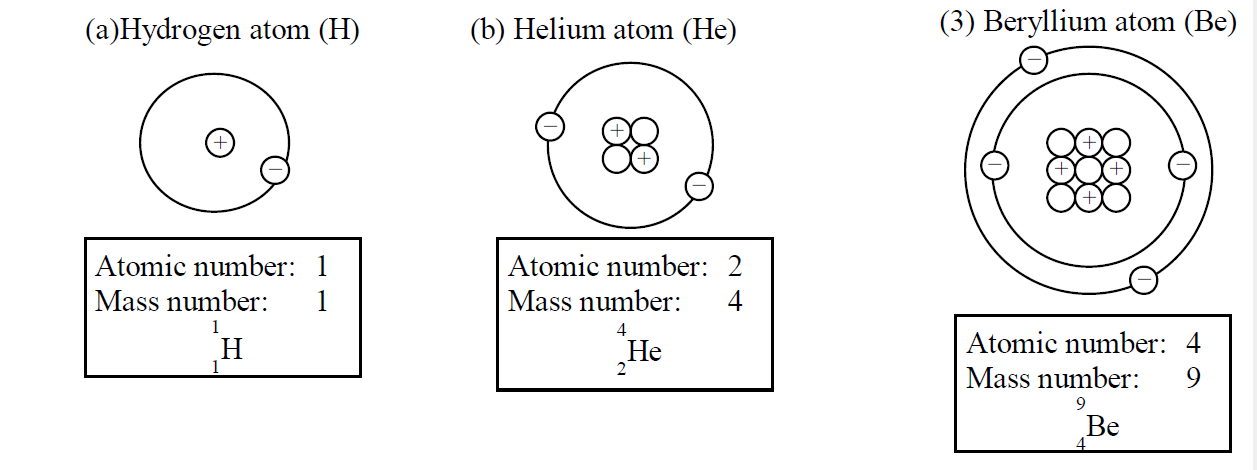
Example elements symbols
Express the following elements by the symbol of
Solution
Nuclide and Isotopes
Each different form of nucleus is called a Nuclide .
Atoms which have the same atomic number but different mass numbers are called Isotopes of an element.
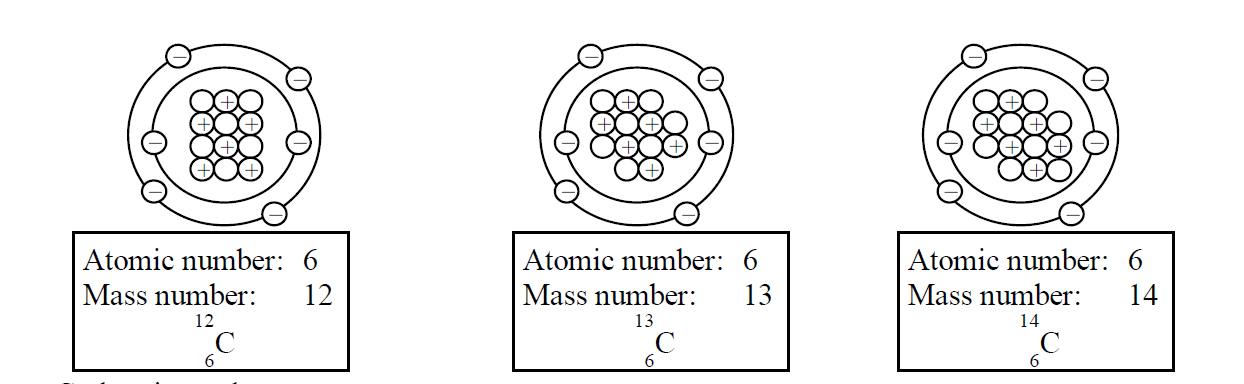
- Carbon is an element.